1. Background
The incidence of bone metastases (BM) in advanced non-small-cell lung cancer (NSCLC) patients is estimated to range from 30% to 40% [1], [2]. The presence of BM often results in pathologic remodeling of the affected bone compartment, making affected bones vulnerable to skeletal related events (SREs). SREs include pathologic fractures, spinal cord compression, requirement for radiation, surgery to bone and hypercalcemia, all reducing quality of life and worsening prognosis [3]. BM is a poor prognostic survival factor [4]. Therefore, early diagnosis and adequate treatment of BM is critically important issues of the clinical management of NSCLC patients.
To detect BM in NSCLC patients, bone scintigraphy combined with plain radiographs, computerized tomography (CT) and magnetic resonance imaging (MRI) is recommended. But routine radiography only gives definite diagnosis when the bone is already substantially damaged by the tumor. Although scintigraphy is more sensitive, its specificity is not satisfactory due to pseudo-positive values caused by inflammation and traumatic fracture. Any abnormal scintigraphic findings should always be verified by radiographic ones [5]. Bone scintigraphy is also a more expensive, invasive, time-consuming, and exposes cancer patients to irradiation, limiting its use for monitoring purposes.
Since BM impairs the balance between bone formation and bone resorption, altered bone remodeling activities can be assessed directly by measuring the components of affected bone cells or indirectly by analyzing metabolic products released from the bone matrix by changed rates of bone formation or resorption. Numerous new analytical tools for bone turnover markers (BTMs) have improved the diagnosis of BM. These BTMs have been recommended as helpful tools for assessing BM [6]. Collectively, there seem to be a diversity of findings depending on cancer type and the type of BTMs used. Previous researches had explored the applications of BTMs in NSCLC patients [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], nevertheless, as to the optimal markers and their proper application in BM screening, there hasn't been a consistent agreement, which greatly hampered the BTMs usage in clinical practice.
Therefore, we (1) measured serum markers of bone formation and bone resorption as noninvasive analytes of bone turnover in NSCLC patients with or without BM, (2) assessed the diagnostic accuracy of these BTMs as potential indicators of BM in NSCLC patients, combined diagnostic effectiveness of BTMs and (3) evaluated with univariate and multivariate analysis of the usefulness of BTMs to make a prognosis in NSCLC patients with BM. We selected bone-specific alkaline phosphatase (BALP), N-terminal midfragment of osteocalcin (N-MID) and aminoterminal propeptide of type I collagen (PINP) as bone formation markers, β-cross-linked carboxyterminal telopeptide of type I collagen (β-CTx) as bone resorption markers.
2. Materials and methods
2.1. Patients and samples
2.1.1. Patients
Our retrospective study included 414 newly diagnosed NSCLC patients that were investigated and treated in the department of internal oncology of the Sixth People's Hospital, Shanghai Jiao Tong University between January 2010 and December 2013. All participants signed approved written consents; the study was done in accordance with the Helsinki Declaration II and Standards of Good Clinical Practice. The Local Ethical Committee has approved the study protocol.
The study consisted of three groups: Group A included 193 NSCLC patients without BM at diagnosis, Group B included 221 NSCLC patients with BM at diagnosis, and Group control included 179 healthy volunteers. The diagnosis of all NSCLC patients was confirmed by histological or cytological examination of specimens taken from bronchoscopy or by CT-guided fine needle biopsy. Cancer stage was assigned according to the TNM system. All patients underwent bone scanning using a radionuclide (Technetium-99m) scintigraphy together with plain radiographs, CT and/or MRI to verify and quantify the presence of BM. In special cases, affected bone lesions by CT-guided fine needle biopsy were used to diagnose BM.
2.1.2. Patients' evaluation
Baseline evaluation included clinical assessment, bone survey, evaluation for extraskeletal disease and serum BTMs determination.
Clinical evaluation included assessment of performance status according to the World Health Organization (WHO) criteria. SREs at diagnosis were recorded, patients were followed up for survival every 3 months and SREs in follow-up were also recorded.
Bone survey included bone scintigraphy and plain radiological, as well as CT or MRI when necessary. Patients were initially classified according to the type and bulk of BM, based on the findings of the bone survey. BM type was characterised as lytic, blastic or mixed. The bulk of BM concerned the number of sites involved and was graded as previously proposed by Soloway [20]. Briefly, Soloway 0 refers to patients without BM; Soloway 1 refers to patients with <6 BM; Soloway 2 refers to patients with <20 BM; Soloway 3 refers to patients with >20 but less than a “super scan”; Soloway 4 refers to patients with “super scan” that is defined by a >75% involvement of the ribs, vertebrae, and pelvic bones.
2.1.3. Samples
Blood samples were collected in plastic tubes between 07:30 and 09:00 a.m., stored in ice and centrifuged at 2000g for 15 min, at 4 °C, within 2 h from venipuncture. Blood samples were collected before the administration of any anticancer treatment after initial diagnosis.
Bone Formation Markers. BALP was determined by the Tandem-MP Ostase Immunoenzymetric Assay (Beckman Colter, Fullerton, CA), which specifically quantifies BALP with low immunoreactivity for the liver/kidney isoforms [21]. N-MID (N-MID-Osteocalcin Assay, Roche) and PINP (Total PINP-Assay, Roche) were measured on the Elecsys 2010 analyzer (Roche). The PINP assay is a new electrochemiluminescent assay that detects both tri- and monomeric PINP forms.
Bone Resorption Markers. β-CTX was determined by the β-CrossLaps Assay (Roche) on the Elecsys 2010 analyzer [22].
2.2. Statistical analysis
Statistical calculations were performed with SPSS® 13 for Windows™ and GraphPad® Prism® 4.03. All results are expressed as mean±SD. We used the nonparametric Kruskal–Wallis ANOVA with Dunn's post test, the Mann–Whitney U test, Spearman's rank correlation coefficients and the Kolmogorov–Smirnov distribution fitting procedure. Diagnostic accuracy was evaluated by Receiver-operating characteristic (ROC) curve analysis. For the combined diagnostic effectiveness of BTMs, the probability was fitted by logistic regression model and then analysed by ROC curves. The Kaplan–Meier product limit method was used to determine survival probability in subgroups. Univariate and multivariate analysis of risk factors predicting NSCLC specific death was performed using the Cox proportional hazards regression model. Differences and associations were considered statistically significant if p<0.05.
3. Results
3.1. Demographics characteristics
221 patients suffered from clinically manifest BM and 33.5% had more than seven BM lesions. In group A, 1 or more metastases in the lung, liver, and other sites (excluding brain) were present in 49.1% patients, while in group B the number is 44.6%. The majority of the patients in both groups received chemotherapy during the study (55.4% in the group A and 80.5% in the group B), and 22.8% in the group A and 29.0% in the group B received target therapy. There were no age or sex difference among the subgroups. For further clinicopathologic data see Table 1, Table 2.
Table 1.
Demographic and Clinicopathologic Characteristics of the Study Groups.
| Characteristics | Control Group | Group A | Group B |
|---|---|---|---|
| Sex | |||
| Female | 84 | 89 | 95 |
| Male | 95 | 104 | 126 |
| Age | 57±11 | 55±9 | 59±10 |
| Tumor stage: | |||
| T1 | — | 23 | — |
| T2 | — | 37 | — |
| T3 | — | 47 | — |
| T4 | — | 86 | 221 |
| Pathologic type | |||
| Adenocarcinoma | — | 91 | 120 |
| Squamous cell carcinoma | — | 28 | 29 |
| Adenosquamous carcinoma | — | 10 | 8 |
| Poor differentiated carcinoma | — | 50 | 53 |
| Alveolar cell carcinoma | — | 6 | 5 |
| Large cell carcinoma | — | 8 | 6 |
| Therapy | |||
| Operation | — | 88 | 2 |
| Chemotherapy | — | 107 | 178 |
| Target therapy | — | 44 | 64 |
| General condition | |||
| ECOG:0-1 | 179 | 159 | 182 |
| ECOG:2 | 0 | 34 | 39 |
Table 2.
Clinic Characteristics of Group B.
| Characteristics | Number |
|---|---|
| The extent of BM | |
| Soloway 1 | 147 |
| Soloway 2 | 65 |
| Soloway 3-4 | 9 |
| Character of BM | |
| Lytic | 153 |
| Blastic | 13 |
| Mixed | 55 |
| Pain level | |
| Mild pain (VAS score:0-3) | 56 |
| Moderate pain (VAS score:4-6) | 141 |
| Severe pain (VAS score:7-10) | 24 |
| With visceral metastases | |
| Yes | 94 |
| No | 127 |
| SREs at diagnosis | |
| Yes | 24 |
| No | 197 |
| SREs in follow-up | |
| Yes | 84 |
| No | 137 |
3.2. Serum BTMs in the study groups
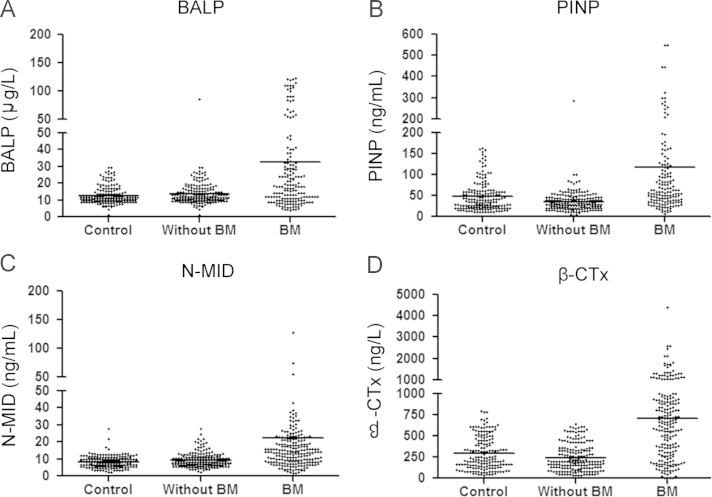
Fig. 1 shows the scatter plots and medians of all BTMs among the subgroups. Since all markers showed a Gaussian distribution using the Kolmogorov–Smirnov test, we calculated the parametric upper 95% reference limits. Briefly, ANOVA analysis showed bone formation BALP, PINP, N-MID and bone resorption β-CTx values were higher in BM patients than in the control group (p<0.05) and in patients without BM (p<0.05), but no difference was found between the control group and the group without BM (p>0.05). For further data see Fig. 1.
Fig. 1.
Scatterplots of bone formation markers (A, B and C) and bone resorption markers (D) in different groups of patients. Horizontal lines indicate median values. BALP, PINP, N-MID and β-CTx values were higher in BM patients than in the control group (p<0.05) and in patients without BM (p<0.05). No differences were found between the control group and the group without BM (p>0.05).
3.3. BTMs as diagnostic Indicators of BM
3.3.1. ROC analyses between NSCLC patients with or without BM
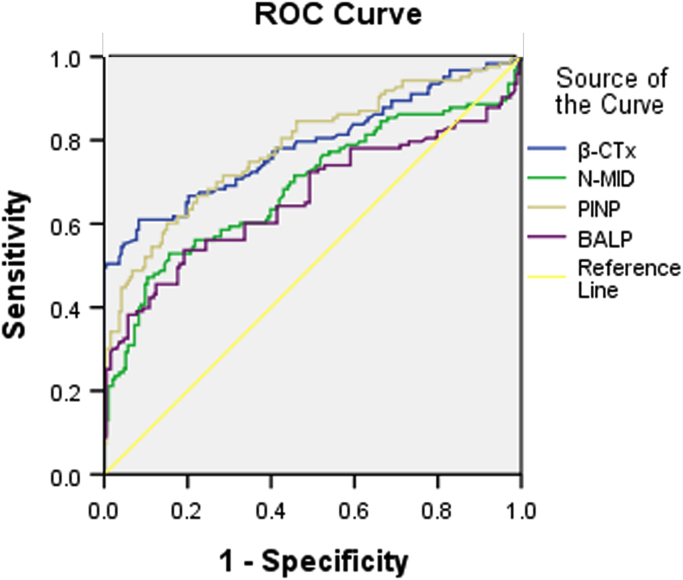
ROC analyses were performed to characterize the diagnostic usefulness of the BTMs, which is to differentiate NSCLC patients with or without BM (Fig. 2 and Table 3). Both bone formation and resorption markers were helpful in this respect. ROC curves were drawn according to the markers based on true-postive ratio (patients with BM) and false-positive ratio (patients without BM). It was obvious that all BTMs were rather effective for this purpose of differentiation. BALP was the most sensitive marker (Area under curve(AUC): 0.787±0.056). Sensitivity and specificity were calculated using cut-off values that correspond to the 95% specificity of the marker tests in the group of NSCLC patients without BM. The cut-off values were found to be 16.96 μg/L for BALP, 52.75 ng/mL for PINP, 12.65 ng/mL for N-MID and 507 ng/L for β-CTx.
Fig. 2.
ROC curves for BTMs to discriminate NSCLC patients with and without BM. Area under curve for BALP was 0.787±0.056, for PINP was 0.688±0.064, for N-MID was 0.782±0.054, and for β-CTx was 0.662±0.067.
Table 3.
Sensitivity, specificity, diagnostic efficiency of BTMs to discriminate NSCLC patients with and without BM.
| Area | 95%CI | Cut-off | SE% | SP% | |
|---|---|---|---|---|---|
| BALP (μg/L) | 0.787 | 0.731–0.843 | 16.96 | 53.7 | 80.8 |
| PINP (ng/mL) | 0.688 | 0.623–0.752 | 52.75 | 60.2 | 85.0 |
| N-MID (ng/mL) | 0.782 | 0.728–0.836 | 12.65 | 52.8 | 84.5 |
| β-CTx (ng/L) | 0.662 | 0.594–0.729 | 507 | 61.0 | 91.7 |
Cut-off values for the calculation of the positivity rates correspond to 95% specificity of the BTMS measurement in patients without BM. p<0.05
3.3.2. ROC curves analysis of the combination of BTMs
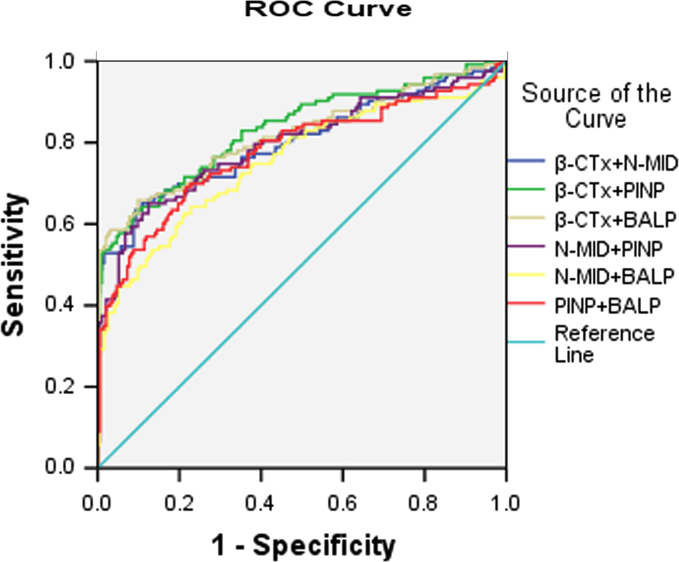
When BTMs were combined for BM screening (Fig. 3 and Table 4), the AUC was elevated. The most effective combination was PINP and β-CTx, to 0.833 (95% CI, 0.785 to 0.882, p<0.0001), and the optimal probability indicated an optimal cut-off value as PINP 40.5 ng/mL and β-CTx 584 ng/L (sensitivity: 61.0%, specificity: 91.2%).
Fig. 3.
ROC curves for combination of BTMs to discriminate NSCLC patients with and without BM. Area under curve for β-CTx+N-MID was 0.802±0.054, for β-CTx+PINP was 0.833±0.049, for β-CTx+BALP was 0.817±0.053, for N-MID+PINP was 0.795±0.055, for N-MID+BALP was 0.753±0.059 and for PINP+BALP was 0.777±0.057.
Table 4.
Sensitivity, specificity, diagnostic efficiency of combination of BTMs to discriminate NSCLC patients with and without BM.
| Combination | Area | 95%CI | Cut-off | SE% | SP% |
|---|---|---|---|---|---|
| β-CTx+N-MID | 0.802 | 0.747–0.856 | β-CTx(664)+N-MID(3.93) | 63.4 | 89.1 |
| β-CTx+PINP | 0.833 | 0.785–0.882 | β-CTx(584)+PINP(40.50) | 61.0 | 91.2 |
| β-CTx+BALP | 0.817 | 0.765–0.870 | β-CTx(508)+BALP(13.00) | 64.2 | 90.2 |
| N-MID+PINP | 0.795 | 0.740–0.850 | N-MID(14.10) +PINP(45.18) | 63.4 | 87.6 |
| N-MID+BALP | 0.753 | 0.694–0.812 | N-MID(8.41)+BALP(25.00) | 61.8 | 78.8 |
| PINP+BALP | 0.777 | 0.721–0.834 | PINP(36.00)+BALP(28.00) | 68.3 | 78.2 |
p<0.05
3.4. Association between BTMs and clinical outcome
Table 5 shows associations between clinical outcome and all BTMs. The demographic data for patients stratified according to Soloway score is indicated in Table 2. There were no linear associations between Soloway score and the demographic characteristics of patients. All BTMs indicated that linear increases with advancing severity of the metastatic involvement of the skeletal system. BALP, PINP and β-CTx were significantly higher in patients with multiple bone site involvement (Soloway 2/3 and 4) than those with few bone site involvement (Soloway 1) (*p<0.05).
Table 5.
Association Between BTMs and Clinical Data.
| Characteristics | BALP (μg/L) | PINP (ng/mL) | N-MID (ng/mL) | β-CTx (ng/L) | P Value |
|---|---|---|---|---|---|
| The extent of BM | ⁎p<0.05 | ||||
| Soloway 1 | 21.31±24.43⁎ | 88.91±140.08⁎ | 23.56±83.5 | 628.84±428.33⁎ | |
| Soloway 2 | 45.06±41.83 | 177.48±256.08⁎ | 19.01±18.57 | 835.84±897.05⁎ | |
| Soloway 3 and 4 | 102.60±152.73⁎ | 133.05±83.71 | 21.19±11.94 | 1022.66±725.13 | |
| Character of BM | p>0.05 | ||||
| Lytic | 30.59±50.34 | 108.07±180.88 | 24.66±82.42 | 695.36±550.33 | |
| Blastic | 24.43±11.57 | 103.14±103.97 | 15.08±10.27 | 603.15±470.64 | |
| Mixed | 38.62±41.63 | 147.08±209.72 | 16.74±10.30 | 758.96±820.62 | |
| With visceral metastases | p>0.05 | ||||
| Yes | 33.80±54.35 | 130.68±231.87 | 21.00±54.02 | 719.17±688.68 | |
| No | 30.45±35.86 | 101.40±101.41 | 23.64±85.18 | 687.65±524.18 | |
| SREs at diagnosis | p>0.05 | ||||
| Yes | 40.63±78.98 | 122.54±201.10 | 23.33±81.10 | 719.30±661.37 | |
| No | 30.02±33.24 | 99.14±106.98 | 21.74±64.67 | 663.90±488.18 | |
| SREs in follow-up | ⁎p<0.05 | ||||
| Yes | 54.09±51.69⁎ | 180.19±283.56⁎ | 23.22±82.36 | 871.70±857.09⁎ | |
| No | 26.64±39.32 | 87.17±97.18 | 19.71±16.25 | 630.44±465.01 |
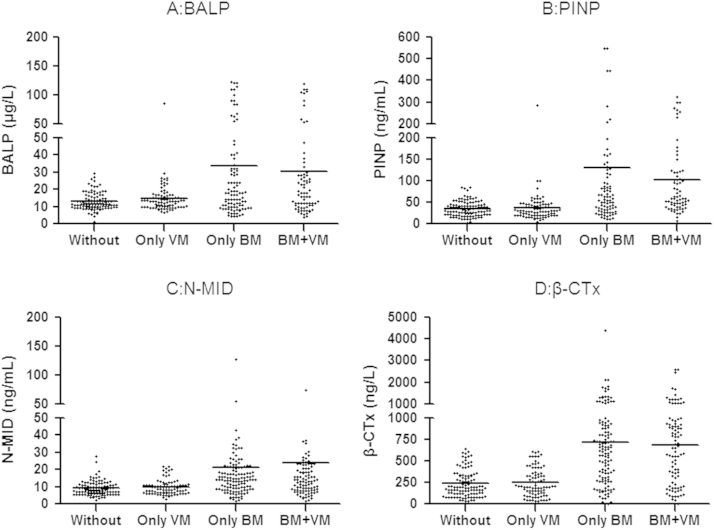
No BTMs have any significant difference among patients with lytic, blastic or mixed bone lesions (p>0.05). Neither bone formation markers nor bone resorption markers differed significantly between patients with bone plus visceral metastases and those with bone metastases only (p>0.05). When patients with BM only or patients with bone and visceral metastases and patients with only visceral metastases were compared with those don't exhibiting metastases, BTM were significantly higher in the two groups with BM with or without visceral metastases (p<0.05). For further data see Fig. 4.
Fig. 4.
Scatterplots of bone formation markers (A, B and C) and bone resorption markers (D) in different groups of patients. Horizontal lines indicate median values. VS: Visceral metastases. BALP, PINP, N-MID and β-CTx values were higher in BM patients with or without BM than in patients without BM (p<0.05). No differences were found between the BM group with or without BM, and no differences were found between the group A with or without BM.
During the study period, SREs occurred in 84 out of 221 patients (38.0%). 61 patients (27.6%) required bone radiation, 27 patients (12.2%) received surgery to bone, 23 patients (10.4%) with pathologic fractures, 8 patients (3.6%) with spinal cord compression and 6 patients (2.7%) with hypercalcemia. Patients with SREs in follow-up had higher β-CTx, BALP or PINP levels compared with patients without SREs (p<0.05), while levels did not differ significantly between patients with or without SREs at diagnosis (p>0.05).
3.5. BTMs as predictors of survival
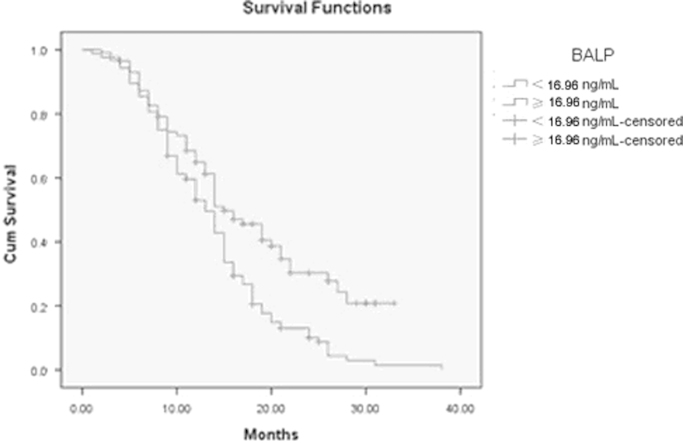
202 of 221 patients were eligible for survival analyses. Median followup was 15 months (range 4 to 40). The primary end point of the analyses was NSCLC related survival. A total of 170 patients died from NSCLC. To determine whether serum BTMs correlate with disease outcome, patients were stratified into 2 groups by analyte cutoff points using the 95th percentiles. To identify the significant prognostic factors associated with NSCLC specific death, univariate and multivariate risk factor analyses were performed using the Cox proportional hazards regression model in the stratified groups (Table 6). BALP, ECOG, Visceral metastases and SREs were significant univariate predictors of death from NSCLC. These results corresponded to Kaplan–Meier survival analysis curves. Patients with BALP above the 95% cutoff, worse ECOG, visceral metastases or SREs had significant shorter survival time than patients with a lower BALP concentration, better ECOG, only bone metastases or without SREs. However, multivariate analysis of the significant predictors showed that BALP, ECOG and SREs were independent predictors of NSCLC related death (Table 6). The median survival time of the patients with normal BALP was 18.0 months, significantly longer than 13.8 months in the patients with elevated BALP (95%CI 12.5–15.1, p<0.05) (Fig. 5). They remained significant variables in the forward and backward stepwise calculation models.
Table 6.
Cox proportional hazards regression univariate and multivariate analysis of serum turnover markers and clinicopathological factors in 211 NSCLC patients with BM.
| Variable | RR | 95% CI | pValue |
|---|---|---|---|
| Univariate: | |||
| BALP | 4.62 | 1.97–10.82 | 0.001 |
| PINP | 2.10 | 0.59–6.88 | 0.27 |
| N-MID | 1.22 | 0.49–3.03 | 0.68 |
| CTx | 1.82 | 0.73–4.56 | 0.21 |
| ECOG | 4.97 | 3.14-7.84 | 0.001 |
| Pathologic type | 1.27 | 0.42–3.75 | 0.69 |
| Character of bone metastases | 2.48 | 0.87–7.29 | 0.12 |
| Soloway score | 2.25 | 0.86–5.85 | 0.09 |
| Visceral metastases | 4.02 | 1.34–11.99 | 0.01 |
| SREs | 11.31 | 2.619–48.91 | 0.001 |
| Multivariate: | |||
| BALP | 2.53 | 1.03–6.30 | 0.04 |
| ECOG | 3.87 | 2.43–6.26 | 0.02 |
| Visceral metastases | 2.12 | 0.68–6.49 | 0.21 |
| SREs | 7.23 | 1.40–33.76 | 0.01 |
Fig. 5.
Kaplan–Meier curves for cancer related survival depending on BALP calculated using Kaplan–Meier method with dichotomized data using log rank test. Criteria for dichotomous classification were 95th percentile cutoff for BALP. The median survival time of the patients with normal BALP was 18.0 months, significantly longer than 13.8 months in the patients with elevated BALP (p<0.05).
4. Discussion
Previous reports evaluating BTMs for the detection of BM in patients with malignant diseases have concluded that urinary N-telopeptide of type I collagen (NTx) is the most useful BTMs [6], [11], [23]. The usefulness of uNTx to diagnose early BM has been reported in several studies [24], [25], [26]. Analyses were performed in both serum and urine, but consistent results were not always described. The pre-analytic variability of BTMs, their different stability in vitro especially in urine and use of assays with different antibodies recognizing different epitopes may explain many of the discrepant results [28], [29], [30], [31]. Seibel [32] indicated serum as the preferred matrix for measurement. He reported the need to measure formation markers (BAP, N-MID, PINP) in serum. Among resorption markers, collagen-derived products can be measured in serum and urine (β-CTx, NTx).
In our opinion, the preferred sample matrix is serum. The use of a single serum for the measure of multiple markers is desirable to simplify pre-analytical and analytical procedures. As commercial assays for measuring BTMs in serum are available, we evaluated the diagnostic value of BTMs in clinical use in detecting BM in NSCLC patients, BALP, PINP and OC, bone formation markers, and CTX, bone resorption markers, which can all be measured in automatically in on sample are assessed. To avoid these discrepancies as far as possible, we measured BTMs exclusively in serum collected during a defined time period, between 7:00 and 9:00 A.M.
The diagnosis of BM in NSCLC patients relies predominantly on imaging techniques. Although these techniques provide useful diagnostic tools, their use for early diagnosis or close monitoring of patients is not without limitations. BTMs as indicators for BM in NSCLC patients have been studied, but there are no clear recommendations which markers or marker combinations should be used [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19]. Kong [22] found that the increased CTX level had the specificity and sensitivity of 65.6% and 68.8%, respectively, in the diagnosis of BM in NSCLC patients, and they speculated that CTX could be used to screen the BM of NSCLC. Ebert [7] found the concentrations of the bone markers BALP, PINP were significantly higher in patients with BM than in those without BM.
In our attempt to document the predictive value of these BTMs in NSCLC patients, we studied a cohort of 414 cancer patients with or without radiographic evidence of BM. We measured the levels of several BTMs in NSCLC patients to assess which BTMs, if any, best reflected the presence of BM. Compared with previous literatures [12], [13], [15], [16], [34], we chose NSCLC patients with no evidence of BM as the control group instead of healthy people. It is known that the balance of bone metabolism is regulated through the action of various systemic hormones and local mediators. We assumed that the formation of tumor in the body, even without evidence of BM, may influence the concentration of those hormones and mediators and result in up-regulation of BTMs. Our results showed the levels of BALP, PINP, N-MID, and β-CTX in the BM group were significantly higher than those in without BM groups. The sample size is considerable and the study provides a valid confirmation of previous reports in smaller studies.
In addition to the clinical validity, both assays were uncomplicated and reliable with good analytic performance; thus, they fulfilled the essential preconditions for routine measurements. The PINP, N-MID and β-CTx assay was an automated assay on a general-purpose analyzer (Elecsys). Meanwhile, BALP could also be automated measured on an analyzer (Access, Beckman–Colter).
Although our work has demonstrated that serum BTMs work well in BM screening, we preferred not to define them as methods for BM diagnosis, nor did we consider that they could replace the screening function of imaging methods. This is because BM is a complicated process, and serum BTMs of an individual patient may be influenced by unknown elements. Serum markers are also unable to fulfill some functions of imaging methods, such as localizing the bone metabolic abnormality.
In our study, the levels of BALP, PINP and β-CTx in the serum were significantly (p<0.05) correlated with the number of skeletal sites involved with metastases, but there was no significant difference in the levels of BTMs among patients with olytic lesions, blastic lesions and mixed lesions. A possible explanation to these findings could be that tumors may secrete these bone regulatory proteins regardless of their location, thus circulating levels maybe elevated even when skeletal involvement has not emerged. Histomorphological results show that osteolytic and osteoblastic metastases are characterized by simultaneous resorptive and osteoblastic processes [6]. The simultaneous increases of bone formation markers and bone absorption markers indicate that the bone formation and bone absorption occur at the same time in patients with BM.
One feature of our research was that we chose two BTMs and combined them for analysis. As the univariate evaluation of data showed dissociated changes of serum marker concentrations for both bone formation and resorptions, multivariate analysis was appropriate. Logistic regression analysis of all variables showed that the combination of β-CTx and PINP was the best way to differentiate between BM and nonmetastases. We assumed that simultaneous elevation of the two BTMs could represent a more robust judgement for BM. Our data was consistent with this assumption: when the bone formation markers and bone resorption markers were used together, the sensitivity and specificity for BM screening were improved as compared with the BTMs being used separately.
Another feature of our research was that we used BTMs clinically to predict the risk of SREs. We found that if patients had a higher β-CTx, BALP or PINP levels at baseline, they were at a higer risk of SREs. A similar pattern was seen in an study [3] evaluating NTx and BALP levels for 441 patients on the placebo arms of the previously noted clinical trials of zoledronic acid in patients with BM from prostate cancer, NSCLC and other solid tumors. Patients with high NTx or BALP levels had a greater incidence of SREs compared with patients with low levels of NTx or BALP. Because survival after diagnosis of BM is relatively short for patients with NSCLC, methods are needed to predict SREs in a shorter timescale than it is possible with current imaging methods. From the results of our current study, the use of BTMs at baseline may make a major contribution to this need by identifying those patients at highest risk who warrant the highest priority for intervention to prevent SREs.
In our study, we evaluated the relationship between serum BTM levels and overall survival (OS) in NSCLC. Correlation analysis revealed that BALP was related to the survival time. Regression analysis showed the number of BM sites, the characteristics of BM (lytic, blastic or mixed), sex and Soloway score did not significantly affect the survival time. When considering the 4 markers that we studied, BALP was the only significant univariate predictor of death from NSCLC. Patients with low serum BALP tend to have longer survival than those with high BALP. The prognostic significance of BALP was verified by multivariate Cox regression analysis since BALP, ECOG and SREs were independent factors of cancer related death. Thus, BALP really appeared to be more a marker of tumor burden or activity than a simple indicator of bone turnover. In addition, the association of BALP with the survival of NSCLC patients could be used for stratifying patients with advanced NSCLC for clinical trials in the current treatment options with bisphosphonates or other chemotherapeutic agents to individualize treatment. For patients with NSCLC, although this analysis cannot address the possibility of a causal link between increases in BTMs and OS, it does suggest that higher BTMs at baseline could have a negative effect on disease progression and might worsen survival.
Some studies assessing the relationship between urinary NTx level and OS in NSCLC have shown that high urinary NTx levels are associated with an increased risk of death [26], [35], [36]. Further, recent studies have suggested that zoledronic acid and denosumab reduce baseline urinary NTx level [25], [36]. Moreover, patients with NSCLC and high baseline NTx levels that were normalized after chemotherapy including zoledronic acid were found to experience longer survival compared with patients whose NTx levels remained persistently high [6], [36], [37]. These study inspired us to find the relation between the change of BTMs and OS. The results of a randomized prospective study would be more credible, and that is what we want to do in the future. But our finding that baseline BTMs levels were predictive of death is also important because it can be argued that baseline BTMs assessments allow more time for appropriate intervention.
5. Conclusion
Our results suggest that, despite of the limitations, measurement of serum BTMs concentration is a powerful test alone or in combination with other BTMs to detect BM and to predict SREs and survival probability in NSCLC patients. However, to fully establish the role of BTMs in clinical practice, prospective studies are needed. Now our suggestion for the proper application of serum BTMs in BM screening is as follows: serum BTMs should be evaluated at once since a patient is diagnosed NSCLC to establish his/her baseline values, and then be monitored regularly and compared with his/her previous results. If the baseline values are higher than the cut-off value, or if abnormal variation is found during the follow up, imaging methods should be applied for further confirmation. If necessary, appropriate intervention should be taken as soon as possible. If the serum BTMs would be used this way, BM screening could become more timely and accurate. As a result, meaningful improvements of life quality and treatment to NSCLC patients may be achieved.
Conflict of interest
None of the authors have identified a conflict of interest.
Acknowledgments
This study was supported by National Natural Science Foundation of China Grant (81201628).
Contributor Information
Zhiyu Wang, Email: areyoufear@163.com.
Yaohong Lu, Email: pbaby322@sina.com.
Dan Qiao, Email: noanoa30@163.com.
Xiaoting Wen, Email: wenxiaoting1@163.com.
Hui Zhao, Email: zhao-hui@sjtu.edu.cn.
Yang Yao, Email: noxfromtheblock@hotmail.com.
References
- 1.Coleman R.E. Skeletal complications of malignancy. Cancer. 1997;80:1588–1594. doi: 10.1002/(sici)1097-0142(19971015)80:8+<1588::aid-cncr9>3.3.co;2-z. [DOI] [PubMed] [Google Scholar]
- 2.Quint L.E., Tummala S., Brisson L.J., Francis I.R., Krupnick A.S., Kazerooni E.A. Distribution of distant metastases from newly diagnosed non-small cell lung cancer. Ann. Thorac. Surg. 1996;62:246–250. doi: 10.1016/0003-4975(96)00220-2. [DOI] [PubMed] [Google Scholar]
- 3.Brown J.E.1, Cook R.J., Major P., Lipton A., Saad F., Smith M. Bone turnover markers as predictors of skeletal complications in prostate cancer, lung cancer, and other solid tumors. J. Natl. Cancer Inst. 2005;97:59–69. doi: 10.1093/jnci/dji002. [DOI] [PubMed] [Google Scholar]
- 4.Al Husaini H., Wheatley-Price P., Clemons M., Shepherd F.A. Prevention and management of bone metastases in lung cancer: a review. J. Thorac. Oncol. 2009;4:251–259. doi: 10.1097/JTO.0b013e31819518fc. [DOI] [PubMed] [Google Scholar]
- 5.Coleman R.E. Monitoring of bone metastases. Eur. J. Cancer. 1998;34:252–259. doi: 10.1016/s0959-8049(97)10134-4. [DOI] [PubMed] [Google Scholar]
- 6.Lipton A., Costa L., Ali S.M., Demers L.M. Bone markers in the management of metastatic bone disease. Cancer Treat Rev. 2001;27:181–185. doi: 10.1053/ctrv.2000.0212. [DOI] [PubMed] [Google Scholar]
- 7.Ebert W., Muley T., Herb K.P., Schmidt-Gayk H. Comparison of bone scintigraphy with bone markers in the diagnosis of bone metastasis in lung carcinoma patients. Anticancer Res. 2004;24:3193–3201. [PubMed] [Google Scholar]
- 8.Lumachi F., Marino F., Fanti G., Chiara G.B., Basso S.M. Serum N-telopeptide of type I collagen and bone alkaline phosphatase and their relationship in patients with nonsmall cell lung carcinoma and bone metastases preliminary results. Anticancer Res. 2011;31:3879–3881. [PubMed] [Google Scholar]
- 9.Kobayashi T., Gabazza E.C., Taguchi O., Risteli J., Risteli L., Kobayashi H. Type I collagen metabolites as tumor markers in patients with lung carcinoma. Cancer. 1999;85:1951–1957. [PubMed] [Google Scholar]
- 10.Chung J.H., Park M.S., Kim Y.S., Chang J., Kim J.H., Kim S.K. Usefulness of bone metabolic markers in the diagnosis of bone metastasis from lung cancer. Yonsei Med. J. 2005;46:388–393. doi: 10.3349/ymj.2005.46.3.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bayrak S.B., Ceylan E., Serter M., Karadağ F., Demir E., Cildağ O. The clinical importance of bone metabolic markers in detecting bone metastasis of lung cancer. Int J. Clin. Oncol. 2012;17:112–118. doi: 10.1007/s10147-011-0266-7. [DOI] [PubMed] [Google Scholar]
- 12.Terpos E., Kiagia M., Karapanagiotou E.M., Charpidou A., Dilana K.D., Nasothimiou E. The clinical significance of serum markers of bone turnover in NSCLC patients: surveillance, management and prognostic implications. Anticancer Res. 2009;29:1651–1657. [PubMed] [Google Scholar]
- 13.Yao N.S., Wu Y.Y., Janckila A.J., Ku C.H., Hsieh A.T., Ho C.L. Serum tartrate-resistant acid phosphatase 5b (TRACP5b) activity as a biomarker for bone metastasis in non-small cell lung cancer patients. Clin. Chim. Acta. 2011;412:181–185. doi: 10.1016/j.cca.2010.09.038. [DOI] [PubMed] [Google Scholar]
- 14.Berruti A., Dogliotti L., Gorzegno G., Torta M., Tampellini M., Tucci M. Differential patterns of bone turnover in relation to bone pain and disease extent in bone in cancer patients with skeletal metastases. Clin. Chem. 1999;45:1240–1247. [PubMed] [Google Scholar]
- 15.Zissimopoulos A., Stellos K., Matthaios D., Petrakis G., Parmenopoulou V., Babatsikou F. Type I collagen biomarkers in the diagnosis of bone metastases in breast cancer, lung cancer, urinary bladder cancer and prostate cancer. Comparison to CEA, CA 15-3, PSA and bone scintigraphy. J. Buon. 2009;14:463–472. [PubMed] [Google Scholar]
- 16.Karapanagiotou E.M., Terpos E., Dilana K.D., Alamara C., Gkiozos I., Polyzos A. Serum bone turnover markers may be involved in the metastatic potential of lung cancer patients. Med. Oncol. 2010;27:332–338. doi: 10.1007/s12032-009-9214-z. [DOI] [PubMed] [Google Scholar]
- 17.Tang C., Liu Y., Qin H., Li X., Guo W., Li J. Clinical significance of serum BAP, TRACP 5b and ICTP as bone metabolic markers for bone metastasis screening in lung cancer patients. Clin. Chim. Acta. 2013;426:102–107. doi: 10.1016/j.cca.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 18.Tamiya M., Kobayashi M., Morimura O., Yasue T., Nakasuji T., Satomu M. Clinical Significance of the Serum Crosslinked N-Telopeptide of Type I Collagen as a Prognostic Marker for Non–Small-Cell Lung Cancer. Clin. Lung Cancer. 2013;14:50–54. doi: 10.1016/j.cllc.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 19.Tamiya M., Tokunaga S., Okada H., Suzuki H., Kobayashi M., Sasada S. Prospective Study of Urinary and Serum Cross-Linked N-Telopeptide of Type I Collagen (NTx) for Diagnosis of Bone Metastasis in Patients With Lung Cancer. Clin. Lung Cancer. 2013;14:364–369. doi: 10.1016/j.cllc.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 20.Soloway M.S., Hardeman S.W., Hickey D., Raymond J., Todd B., Soloway S. Stratification of patients with metastatic prostate cancer based on extent of disease on initial bone scan. Cancer. 1988;16:195–202. doi: 10.1002/1097-0142(19880101)61:1<195::aid-cncr2820610133>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 21.Broyles D.L., Nielsen R.G., Bussett E.M., Lu W.D., Mizrahi I.A., Nunnelly P.A. Analytical and clinical performance characteristics of Tandem-MP Ostase, a new immunoassay for serum bone alkaline phosphatase. Clin. Chem. 1998;44:2139–2147. [PubMed] [Google Scholar]
- 22.Okabe R., Nakatsuka K., Inaba M., Miki T., Naka H., Masaki H. Clinical evaluation of the Elecsys betaCrossLaps serum assay, a new assay for degradation products of type I collagen C-telopeptides. Clin. Chem. 2001;47:1410–1414. [PubMed] [Google Scholar]
- 23.Izumi M., Nakanishi Y., Takayama K., Kimotsuki K., Inoue K., Wataya H. Diagnostic value of bone-turnover metabolites in the diagnosis of bone metastases in patients with lung carcinoma. Cancer. 2001;91:1487–1493. doi: 10.1002/1097-0142(20010415)91:8<1487::aid-cncr1156>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 24.Sugiura H., Yamada K., Sugiura T., Hida T., Mitsudomi T. Predictors of survival in patients with bone metastasis of lung cancer. Clin. Orthop. Relat. Res. 2008;466:729–736. doi: 10.1007/s11999-007-0051-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Henry D.H., Costa L., Goldwasser F., Hirsh V., Hungria V., Prausova J. Randomized, double-blind study of denosumab versus zoledronic acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. J. Clin. Oncol. 2011;29:1125–1132. doi: 10.1200/JCO.2010.31.3304. [DOI] [PubMed] [Google Scholar]
- 26.Hirsh V., Major P.P., Lipton A., Cook R.J., Langer C.J., Smith M.R. Zoledronic acid and survival in patients with metastatic bone disease from lung cancer and elevated markers of osteoclast activity. J. Thorac. Oncol. 2008;3:228–236. doi: 10.1097/JTO.0b013e3181651c0e. [DOI] [PubMed] [Google Scholar]
- 28.Plebani M., Bernardi D., Meneghetti M.F., Ujka F., Zaninotto M. Biological variability in assessing the clinical value of biochemical markers of bone turnover. Clin. Chim. Acta. 2000;299:77–86. doi: 10.1016/s0009-8981(00)00285-0. [DOI] [PubMed] [Google Scholar]
- 29.Hannon R., Eastell R. Preanalytical variability of biochemical markers of bone turnover. Osteoporos. Int. 2000;11:S30–S44. doi: 10.1007/s001980070004. [DOI] [PubMed] [Google Scholar]
- 30.Schober E.A., Breusch S.J., Schneider U. Instability and variability of urinary telopeptides and free crosslinks. Clin. Chim. Acta. 2002;324:73–79. doi: 10.1016/s0009-8981(02)00208-5. [DOI] [PubMed] [Google Scholar]
- 31.Woitge H.W., Pecherstorfer M., Li Y., Keck A.V., Horn E., Ziegler R. Novel serum markers of bone resorption: clinical assessment and comparison with established urinary indices. J. Bone Miner. Res. 1999;14:792–801. doi: 10.1359/jbmr.1999.14.5.792. [DOI] [PubMed] [Google Scholar]
- 32.Seibel M.J. Biochemical markers of bone turnover. Part I: biochemistry and variability. Clin. Biochem. Rev. 2005;26:97–122. [PMC free article] [PubMed] [Google Scholar]
- 34.Schindler F., Lajolo P.P., Pinczowski H., Fonseca F.L., Barbieri A., Massonetto L.H. Bone and total alkaline phosphatase for screening skeletal metastasis in patients with solid tumours. Eur. J. Cancer Care (Engl) 2008;17:152–156. doi: 10.1111/j.1365-2354.2007.00826.x. [DOI] [PubMed] [Google Scholar]
- 35.Mountzios G., Ramfidis V., Terpos E., Syrigos K.N. Prognostic significance of bone markers in patients with lung cancer metastatic to the skeleton: a review of published data. Clin. Lung Cancer. 2011;12:341–349. doi: 10.1016/j.cllc.2011.03.032. [DOI] [PubMed] [Google Scholar]
- 36.Kaira R., Murakami H., Kaira K., Takahashi T., Tsuya A., Nakamura Y. N-telopeptide of type I collagen is useful for monitoring therapeutic response in non-small cell lung cancer patients with bone metastases. Int. J. Clin. Oncol. 2010;15:484–488. doi: 10.1007/s10147-010-0100-7. [DOI] [PubMed] [Google Scholar]
- 37.Lipton A., Costa L., Ali S., Demers L. Use of markers of bone turnover for monitoring bone metastases and the response to therapy. Semin. Oncol. 2001;28:54–59. doi: 10.1016/s0093-7754(01)90233-7. [DOI] [PubMed] [Google Scholar]