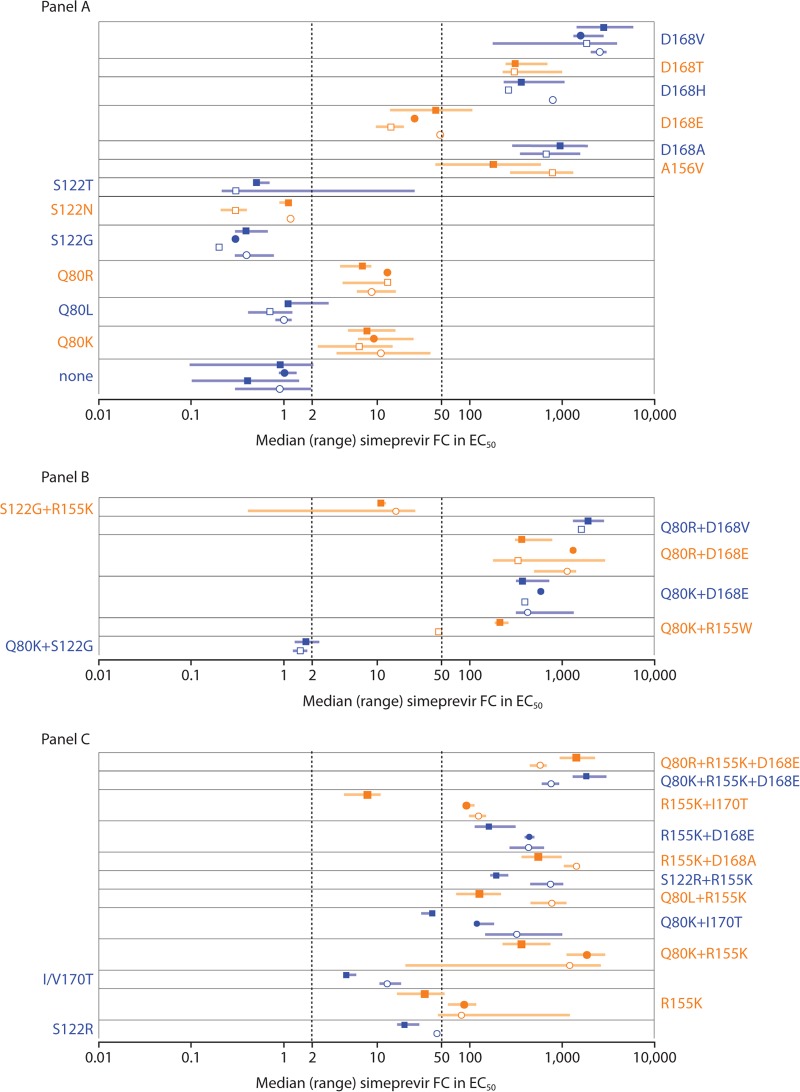
FIG 4.
Activity of simeprevir against HCV genotype 1a and genotype 1b NS3 isolates carrying no, single (A), double, and triple (B) amino acid substitutions at NS3 positions 80, 122, 155, 156, 168, and/or I/V170T and against corresponding SDMs in genotype 1a and genotype 1b replicon backbones. (C) Data for isolates and SDMs with differences in simeprevir susceptibility by HCV geno/subtype. Open circles (○) and boxes (□), median simeprevir FC values of genotype 1a and 1b isolates, respectively; filled circles (●) and boxes (■), median simeprevir FC values of SDMs in genotype 1a and 1b replicon backbones, respectively. Error bars represent the range. Simeprevir FC values for individual clinical isolates were calculated as median values across 1 to 7 replicates (the majority of isolates [341 of 435] were tested ≥3 times). Clinical isolates containing the same single, double, triple, or no amino acid substitutions at NS3 positions 43, 80, 122, 155, 156, 168, and/or 170 were grouped, and the median FC value for each group was determined (each isolate group contained 1 to 95 individual isolates; for details, see Table S5 in the supplemental material). Simeprevir FC values for SDMs carrying corresponding simeprevir resistance-associated amino acid substitutions were calculated as median values across 1 to 24 experimental replicates (the majority of SDMs [38 of 41] were tested ≥3 times). EC50, 50% effective concentration; FC, fold change; HCV, hepatitis C virus; SDM, site-directed mutant.