Abstract
Pyrazinamide (PZA) is active against major Mycobacterium tuberculosis species (M. tuberculosis, M. africanum, and M. microti) but not against M. bovis and M. avium. The latter two are mycobacterial species involved in human and cattle tuberculosis and in HIV coinfections, respectively. PZA is a first-line agent for the treatment of human tuberculosis and requires activation by a mycobacterial pyrazinamidase to form the active metabolite pyrazinoic acid (POA). As a result of this mechanism, resistance to PZA, as is often found in tuberculosis patients, is caused by point mutations in pyrazinamidase. In previous work, we have shown that POA esters and amides synthesized in our laboratory were stable in plasma (M. F. Simões, E. Valente, M. J. Gómez, E. Anes, and L. Constantino, Eur J Pharm Sci 37:257–263, 2009, http://dx.doi.org/10.1016/j.ejps.2009.02.012). Although the amides did not present significant activity, the esters were active against sensitive mycobacteria at concentrations 5- to 10-fold lower than those of PZA. Here, we report that these POA derivatives possess antibacterial efficacy in vitro and ex vivo against several species and strains of Mycobacterium with natural or acquired resistance to PZA, including M. bovis and M. avium. Our results indicate that the resistance probably was overcome by cleavage of the prodrugs into POA and a long-chain alcohol. Although it is not possible to rule out that the esters have intrinsic activity per se, we bring evidence here that long-chain fatty alcohols possess a significant antimycobacterial effect against PZA-resistant species and strains and are not mere inactive promoieties. These findings may lead to candidate dual drugs having enhanced activity against both PZA-susceptible and PZA-resistant isolates and being suitable for clinical development.
INTRODUCTION
Mycobacterium tuberculosis is a particularly successful pathogen which latently infects about 2 billion people, almost one-third of the world's population (1). Each year, there are about 8 million new cases of tuberculosis (TB) and 1.3 million deaths worldwide. Fifty million people have already been infected with drug-resistant TB (1). This situation arose from (i) the spread of HIV worldwide, a virus that weakens the immune system, allowing the reactivation of latent drug-susceptible or drug-resistant M. tuberculosis strains, and (ii) noncompliance, often due to toxic side effects during the long-lasting treatment.
Pyrazinamide (PZA) is an important frontline anti-TB drug that plays a key role in shortening TB therapy from 9 to 12 months to the current 6 months (2). The ability of PZA to shorten TB therapy is due to its activity against a population of nongrowing, persistent tubercle bacilli residing in an acidic pH phagosomal environment that is not killed by other anti-TB drugs (3–5). Furthermore, studies have shown that a regimen of PZA combined with rifampin for 2 months is as effective at preventing TB in HIV carriers as a 6-month isoniazid treatment (6).
The Cynamon group postulated in 1992 that pyrazinamide was converted to pyrazinoic acid (POA) within the bacterial lumen and that POA was the active antimycobacterial agent (7). Indeed, PZA is a prodrug whose activation requires hydrolysis by the pyrazinamidase enzyme encoded by the pncA gene of M. tuberculosis (2, 3). The unique susceptibility of M. tuberculosis to PZA is at least partly due to an inefficient efflux mechanism that allows POA to be increasingly accumulated in the acidic interior of M. tuberculosis (2).
While acquired resistance to PZA in M. tuberculosis occurs mainly by mutations in the pncA gene (8), the mechanism of natural PZA resistance in other mycobacteria is more complex. Like M. tuberculosis, M. bovis strains, including BCG, are naturally resistant to PZA due to a single point mutation (C→G) at nucleotide position 169 in pncA (9). In contrast, naturally PZA-resistant M. smegmatis and other bacteria, such as Escherichia coli, have a highly active POA efflux mechanism that prevents the accumulation of POA even at acidic pH (10). M. avium has high pyrazinamidase activity, and its natural resistance to PZA probably is due to the POA efflux mechanism (11).
If PZA is a prodrug, then it would be possible to develop alternative prodrugs that deliver POA intracellularly. This hypothesis was successfully developed by the work of Cynamon et al. in a series of papers dedicated to exploring the potential of such esters as antituberculosis agents (7, 12, 13). The advantage of these compounds seems evident; because esterases are abundant in mycobacteria, prodrugs can be easily activated in situ. Moreover, resistant strains will hardly be selected, as the mechanism would no longer depend on a single gene point mutation. Esterases also exist in human plasma; therefore, these esters may be hydrolyzed before reaching their bacterial target, their active moiety (POA) being too polar for effective permeation into mycobacteria (12–14).
In order to improve the stability of the POA esters, we designed and synthesized several long-chain esters, showing that esters with long alkoxy chains are particularly resistant to hydrolysis in the plasma (14). This fact suggested that it is possible to identify some POA ester prodrugs with increased resistance toward plasmatic hydrolysis. Here, we demonstrate that such POA ester prodrugs are active against PZA-resistant M. tuberculosis isolates as well as against naturally PZA-resistant species of mycobacteria, such as M. bovis and M. avium. Our results indicate that their resistance was overcome by two factors, the release of POA by enzymes other than pyrazinamidase and the intrinsic antimycobacterial effect of long-chain alkanols.
The new compounds were shown to possess in vitro and ex vivo activities within macrophages infected with sensitive strains of M. tuberculosis or M. avium even at concentrations 10- to 100-fold lower than those of PZA.
MATERIALS AND METHODS
Materials.
Balanced salt solution, phosphate-buffered saline (PBS), Dulbecco's modified Eagle's medium (DMEM), and l-glutamine were purchased from Invitrogen. Sodium dodecyl sulfate (SDS), Triton X-100, pyrazinamide (PZA), pyrazinoic acid (POA), n-dodecanol (12-ol), n-tetradecanol (14-ol), n-hexadecanol (16-ol), n-butanol (4-ol), and trypan blue were purchased from Sigma-Aldrich Quimica SA. Middlebrook 7H10 agar was purchased from Difco. The materials and equipment used for antibiotic susceptibility tests of M. tuberculosis with the Bactec MGIT 960 PZA kit system were purchased from Becton Dickinson and prepared according to the recommendations of the manufacturer. Microwell tissue culture plates were purchased from Nunc. Pyrazinamide and the esters of POA, namely, E-12 (n-dodecyl pyrazinoate), E-14 (n-tetradecyl pyrazinoate), and E-16 (n-hexadecyl pyrazinoate), were synthesized as described previously (14). They then were prepared in stock solutions of 8 mg/ml in dimethyl sulfoxide (DMSO), diluted in Middlebrook 7H9 medium containing ADC (albumin, dextrose, and catalase; oleic acid was not included, as it is toxic for mycobacteria at acidic pH [15]; Difco), and acidified to pH 5.9 (14).
Bacterial strains.
M. tuberculosis H37Rv (ATCC 27294), a PZA-susceptible strain, served as the positive control; five clinical strains with acquired resistance to PZA (multidrug-resistant [MDR]-TB) and maintained in the Unit of Bacteriology, Health Institute of Lisbon, Lisbon, Portugal, were used as M. tuberculosis PZA-resistant strains (16). Two out of the five clinical strains were selected for their susceptibility to POA. M. bovis isolated from infected cattle was provided by the National Laboratory for Veterinary Research, Lisbon, Portugal. M. bovis BCG (CIP 105050) was purchased from the Pasteur Institute, Pasteur Collection, Paris, France, and the two M. avium strains, DZMC 44156 and DZMC 44157, were from the German Collection (17). M. smegmatis ATCC 607 variant mc2155 was used for the production of the homogenate.
Activation of the prodrugs in M. avium and BCG homogenates.
M. avium homogenate and BCG homogenate were prepared using the technique described for M. smegmatis (14, 18). The incubation of the homogenate with prodrug was performed at 37°C in a total volume of 1,500 μl. The concentration of substrate in incubation media was 1.2 × 10−4 M, and the protein concentration was 0.12 mg/ml. Dilutions were performed using PBS. At predetermined time points, aliquots of 150 μl were taken into vials containing 300 μl of acetonitrile and 300 μl of a 1% ZnCl2 aqueous solution. The suspension then was agitated in a vortex and centrifuged for 5 min. The supernatant was injected into a high-performance liquid chromatographer (HPLC), as described previously (14), for quantification of the prodrug and POA.
Determination of the MICs.
The MICs of PZA, POA, the long-chain ester prodrugs of POA (E-12, E-14, and E-16), and the corresponding long-chain fatty alcohols used as promoieties (12-ol, 14-ol, and 16-ol) were determined individually by two methods, the broth dilution method, available in our laboratory (19), and the Bactec MGIT 960 PZA kit provided by the National Health Institute, as described previously (16). Briefly, five MDR-TB isolates and the susceptible M. tuberculosis, used as a reference, were tested for susceptibility to PZA by the Bactec MGIT 960 PZA kit at a reduced pH of 5.9 and a 100 μg/ml concentration of PZA (Becton Dickinson) according to the manufacturer's manual. We retested these five MDR-TB strains for PZA susceptibility by using the Bactec MGIT 960 PZA with 100, 300, and 900 μg/ml concentrations of PZA. In parallel, we used the dilution method, screening drug concentrations ranging from 5 to 1,000 μg/ml for the same M. tuberculosis strains tested by the Bactec system for M. bovis, M. bovis BCG, and M. avium. The acidified Middlebrook 7H9 medium (pH 5.9) was supplemented with ADC (Difco) (14) and an adjusted concentration of mycobacteria (approximately 105 CFU). Each agent or prodrug screened in serial dilutions was incubated for 10 days at 37°C. For the long-chain alkanols, the pH was adjusted to 7 as usual for compounds other than POA prodrugs. The MIC was the minimum drug concentration where no visible turbidity was observed. The assay was extended until day 30. A test tube without drug was used as the control. The MIC values obtained were similar in the two methods.
Macrophage cell line J774A.1.
The J774 macrophage cell line was kindly provided by Gareth Griffiths, EMBL, Heidelberg, Germany, and was maintained as described previously (17).
Macrophage infection and ex vivo intracellular killing activity.
Bacterial cultures in the exponential growth phase were pelleted, washed twice in PBS (pH 7.4), and resuspended in DMEM to a final optical density at 600 nm (OD600) of 0.1, corresponding to 107 colony-forming microorganisms per ml. Clumps of bacteria were removed by ultrasonic treatment of bacterial suspensions in an ultrasonic water bath for 15 min, followed by low-speed centrifugation at 120 × g for 2 min. Single-cell suspension was verified by light microscopy. J774 cells were seeded onto 24-well tissue culture plates at a density of 0.5 × 105 cells per well and were incubated for 2 days until 80% confluence and infected with bacteria at a multiplicity of infection of 10:1. After 3 h of bacterial internalization by macrophages at 37°C and 5% CO2, remaining extracellular bacteria were removed by intensive washing with PBS. The prodrugs then were added to the medium and incubated for 7 days. Every second day, the medium was replaced with fresh medium containing the test compound.
At the end of the bacterial internalization by macrophages (3 h) and after 1, 3, 5, and 7 days postinfection, macrophages were washed with PBS and disrupted with 1% IGEPAL CA-630 (Sigma-Aldrich) solution in water, a nonionic, nondenaturing detergent that disrupts eukaryotic cells but does not affect mycobacterial viability, with the goal being to assess the colony-forming microorganisms of viable intracellular bacteria.
Serial dilutions of the macrophage culture lysate were prepared in water and plated in Middlebrook 7H10 agar medium supplemented with oleic acid-albumin-dextrose-catalase (OADC; Difco). After about 3 weeks of incubation at 37°C, colonies were counted. The proportion of surviving mycobacteria in samples treated with the compounds was compared to controls of infected cells at the same time point treated with the same dilution of the dilution solvent (mock control) and was taken as a measure of the in vivo activity of the screened compounds. The assays were done in triplicate and from independent experiments.
Cytotoxicity assay.
J774A.1 cells were incubated with the compounds at concentrations of 20 μg/ml for esters E-12, E-14, and E-16 and at 5 and 10 μg/ml for the n-alkanols 12-ol, 14-ol, and 16-ol for 5 days. Culture media with the compounds was replenished every second day during the course of the experiment. DMSO, at the same proportions as those in the tested compounds, was used as a control. The highest concentration of DMSO used was 1%. Puromycin (Sigma-Aldrich) was used as a positive control for cell death. Cell viability was determined after 5 days of treatment using alamarBlue (Molecular Probes) by following the manufacturer's indications. Briefly, 10% (vol/vol) alamarBlue reagent was added to each well and incubated for 4 h at 37°C and 5% CO2. Fluorescence was measured at an excitation of 570 nm and emission of 595 nm in a TecanM200 plate spectrophotometer. Viability was calculated as percent fluorescence intensity relative to that of the untreated controls. Test results were obtained from at least four independent experiments, each performed in triplicate.
Statistical analysis.
All values are reported as means ± standard deviations (SD) from 3 independent experiments. The statistical significance of the differences observed in bacterial loads was analyzed by the Student t test. Differences were considered significant at P < 0.05 (*) or P < 0.01(**).
RESULTS
Hydrolysis of the esters.
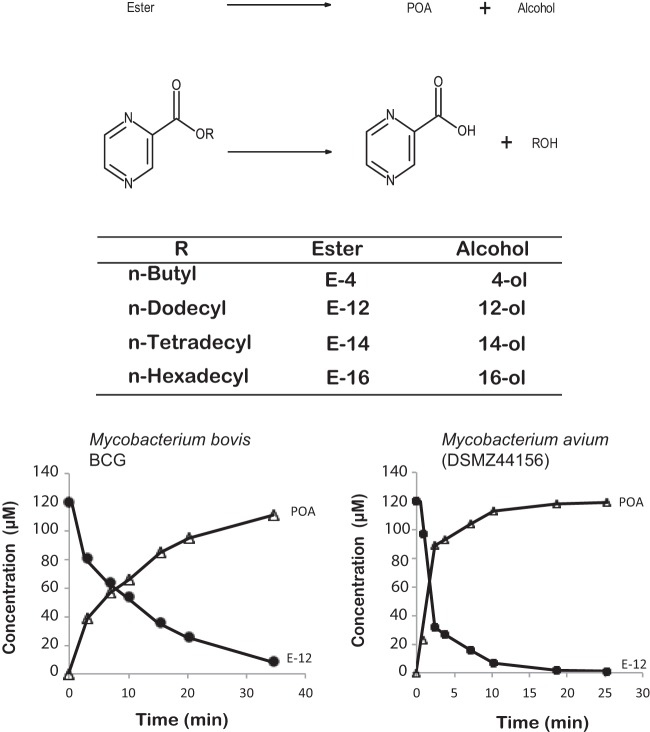
Figure 1 shows the structures of the esters and alcohols used in this study and also the hydrolysis reaction to POA and the corresponding alcohol. The incubation of the esters with mycobacterial homogenate (BCG or M. avium) gives rise to POA and the corresponding alcohol. As can be seen from Fig. 1, the disappearance of the ester corresponds quantitatively to the formation of POA.
FIG 1.
Hydrolysis reaction of pyrazinoate esters to POA and the corresponding alcohol and structure of the compounds studied. The concentrations of ester E-12 and POA during the hydrolysis of E-12 in M. bovis BCG or M. avium homogenates are shown.
Effect of PZA, POA, and POA esters on PZA-susceptible and -resistant mycobacteria in vitro.
The calculated MICs of PZA, POA, and POA esters (PAEs) are summarized in Table 1 for a pH of the medium adjusted to 5.9. As expected, the results for PZA are in agreement with the literature, with a MIC of 10 to 20 μg/ml at pH 5.5 (20) and 100 μg/ml at pH 5.9 (16, 21). By using exactly the same conditions, all three POA esters displayed a 5-fold lower MIC than PZA or POA in vitro against the M. tuberculosis-susceptible strain.
TABLE 1.
MICs of POA esters POA and PZA in vitro
| Compounda | MICb (μg/ml) |
||||||
|---|---|---|---|---|---|---|---|
| M. bovis BCG | M. bovis | M. avium 44156 | M. avium 44157 | M. tuberculosis H37Rv | M. tuberculosis PZAR1 | M. tuberculosis PZAR2 | |
| E-12 | 20 | 40 | 20 | 10 | 20 | 40 | 20 |
| E-14 | 20 | 40 | 20 | 10 | 20 | 40 | 20 |
| E-16 | >40 | >40 | 40 | 40 | 20 | >40 | >40 |
| PZA | >1,000 | >1,000 | >1,000 | >1,000 | 100 | >1,000 | >1,000 |
| POA | 100 | 100 | >1,000 | >1,000 | 100 | 100 | 100 |
E-12, n-dodecyl pyrazinoate; E-14, n-tetradecyl pyrazinoate; E-16, n-hexadecyl pyrazinoate; PZA, pyrazinamide; POA, pyrazinoic acid.
rM. tuberculosis H37Rv is the reference sensitive strain. All other species and strains are resistant to PZA.
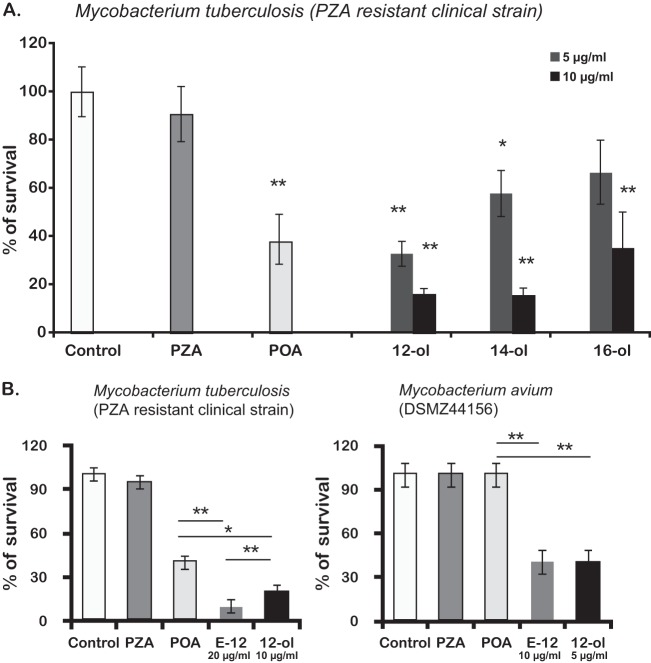
When PZA-resistant strains of M. tuberculosis or naturally resistant species, such as M. avium and M. bovis, including BCG, were used to screen the new agents, the results were as depicted in Table 1. While PZA has a MIC higher than 1,000 μg/ml for all strains and species tested, confirming their resistance, the esters E-12 and E-14 (obtained from 12-ol and 14-ol, respectively) showed MICs of 10 to 40 μg/ml, indicating their efficacy against PZA-resistant mycobacteria. For E-16 (n-hexadecyl pyrazinoate), the MIC was slightly higher than those for E-12 and E-14 but still two orders of magnitude lower than those of PZA against resistant mycobacteria.
The results also indicate that POA, with a MIC similar to that of PZA in sensitive strains, is active against PZA-resistant mycobacteria except M. avium. Indeed, the differences observed between M. tuberculosis H37Rv and the M. tuberculosis PZA-resistant strain with sensitivity to POA indicates that the resistance on these M. tuberculosis clinical strains is due to a mutation in pyrazinamidase, as confirmed by DNA sequencing of the pncA gene (16). For all M. avium strains tested, resistance against PZA and, indeed, POA was detected with MICs higher than 1,000 μg/ml. However, all POA esters (E-12 to E-16) were shown to possess antimycobacterial activity against M. avium, with MICs ranging from 10 to 40 μg/ml.
Antibacterial effects of the pyrazinoate esters on susceptible and resistant mycobacteria in infected macrophages.
We first determined the MIC of each PAE against an intracellular M. tuberculosis susceptible strain. For each compound we selected a range of concentrations above and below the in vitro MIC, ranging from 10 to 200 μg/ml. Figure 2A shows the antimycobacterial effects on the susceptible strain within J774 macrophages. The percentage of intracellular survival is represented at days 0, 1, 3, 5, and 7. The culture medium with the drug was added 3 h postbacterial internalization by macrophages and was replaced every second day. For PZA and POA, the ex vivo active concentration was 100 μg/ml, while for the PAEs E-12, E-14, and E-16 it was 20 μg/ml, confirming an ex vivo MIC similar to that obtained in vitro. Lower concentrations of these compounds were ineffective in infected macrophages. On day one postinfection, all of the compounds were effective against M. tuberculosis. E-12 and E-14, at a concentration 5-fold lower than that of PZA or POA, presented an approximately 50% higher antimycobacterial activity than that of PZA and POA (Fig. 2A). This activity was maintained throughout the experiment. At day seven postinfection, the activity of the esters was significantly more effective against intracellular mycobacteria than PZA or POA (Fig. 2A).
FIG 2.
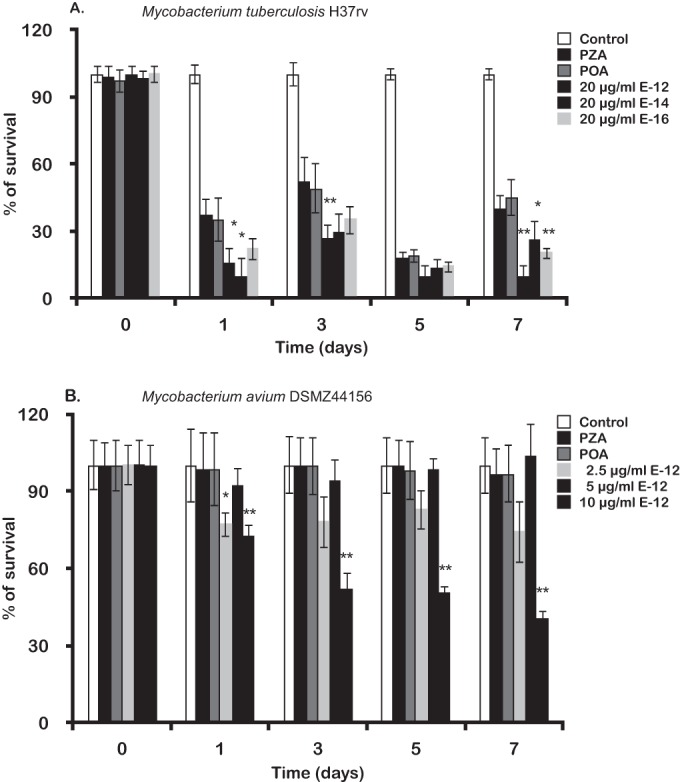
Ex vivo antibacterial effects of POA esters on mycobacteria. The ordinate represents the percentage of remaining intracellular survival measured by CFU/ml along 7 days postinfection. (A) Effects of E-12, E-14, and E-16 on intracellular M. tuberculosis compared to those of PZA and POA. (B) Effect of E-12 on M. avium within J774 macrophages. PZA and POA were used to confirm the natural resistance of M. avium to these compounds. The statistical significance of the differences observed in bacterial loads was analyzed by the Student t test. Differences were considered significant at P < 0.05 (*) or P < 0.01 (**).
Since ester E-12 had higher activity against intracellular susceptible M. tuberculosis and a MIC of 10 to 20 μg/ml against two strains of M. avium, we selected this compound to determine its activity against intracellular M. avium. Figure 2B shows how its activity evolved during the days following infection, emphasizing its effects on intracellular survival rate compared to those of PZA and POA. As the MIC determined in vitro for M. avium DZMC 44156 was 20 μg/ml at pH 5.9, we chose to test three concentrations below the MIC in infected macrophages. The concentration found to be effective was 10 μg/ml with a 50% decrease of intracellular M. avium from day 3 postinfection relative to those of PZA- or POA-treated cells (Fig. 2B).
Mycobacterium avium is naturally resistant to most antibiotics, including PZA. Unlike M. tuberculosis, whose PZA resistance is due to mutations in the pncA gene, M. avium has high PZase activity. M. avium natural resistance to PZA most likely is due to a POA efflux mechanism, but this mechanism has not been fully proven so far (11). The MICs shown in Table 1 clearly suggest that M. avium is resistant to POA, in contrast to the other tested mycobacteria. This led us to investigate the effect of these POA esters against the two M. avium reference strains. Incubation of esters with an M. avium homogenate gives rise to quantitative hydrolysis of the POA esters to POA (Fig. 1). Because POA did not present activity when tested against M. avium (Table 1), we decided to test the liberated long-chain alcohols, as these are likely candidates to account for antimycobacterial activity. Therefore, we examined alcohols with alkyl chain lengths from C4 to C16 and determined their MICs against different mycobacteria strains, including M. avium, as depicted in Table 2.
TABLE 2.
MICs of the n-alkanols in vitro
| Compound | MIC (μg/ml) |
|||
|---|---|---|---|---|
| M. bovis BCG | M. avium 44156 | M. avium 44157 | M. tuberculosis H37Rv | |
| 4-ola | >80 | >80 | >80 | 10 |
| 12-ol | 10 | 40 | 40 | 5 |
| 14-ol | 5 | 20 | 20 | 5 |
| 16-ol | 5 | 20 | 20 | 5 |
| Kanamycin | 0.5 | 10 | 10 | 1 |
4-ol (butanol) was used to estimate the effect of a short-chain alcohol.
As expected, all long-chain alcohols displayed an antimycobacterial activity slightly higher than that of POA esters, with MICs ranging from 5 to 40 μg/ml and efficacy increasing with chain length.
Interestingly, Kushner et al. (22) obtained relevant antimycobacterial activity with ethyl thiopyrazinoate and also found that ethanethiol, the potential liberated thiol, presented very good activity (even higher than that of the ester) when tested alone, shifting the focus of their research from the pyrazine nucleus to thiol moieties.
Antibacterial effect of the long-chain alcohols against intracellular mycobacteria.
We next tested the effect of long-chain alkanols on the intracellular survival of PZA-resistant mycobacteria. Effects of the long-chain alcohols on the intracellular survival of M. tuberculosis in macrophages were determined first. The greatest effects on bacterial killing were observed after 5 or more days of incubation. The results shown in Fig. 3 show that the 12-carbon alcohol was most effective at killing the bacteria at 5 days postinfection. Although the 12-carbon alcohol usually was less effective on most strains in broth culture than its homologs, inside macrophages the 12-ol was very effective. The PZA-resistant strain of M. tuberculosis demonstrated comparable sensitivities to 100 μg/ml POA and to 12-ol at 10 μg/ml. No differences were observed between the M. tuberculosis reference strain H37Rv relative to the PZA-resistant strain for all compounds with the exception of PZA (compare Fig. 2A, day seven postinfection, to Fig. 3B, left).
FIG 3.
Ex vivo antibacterial effects of long-chain alkanols and corresponding E-12 ester on PZA-resistant mycobacteria. (A) Effects on intracellular M. tuberculosis of long-chain alkanols. (B) Effects on M. tuberculosis and on M. avium of 12-ol compared with those of PZA and POA (100 μg/ml) and the corresponding PZA ester E-12. The statistical significance of the differences observed in bacterial loads was analyzed by the Student t test. Differences were considered significant at P < 0.05 (*) or P < 0.01 (**).
In this context, n-dodecanol again proved to be the most active n-alkanol screened. We then compared the effect of 12-ol to that of the corresponding POA ester E-12 on the intracellular killing of the M. tuberculosis PZA-resistant strain and/or M. avium DZMC44156 7 days postinfection. The results (Fig. 3B, right) show that comparable effects were caused by 10 μg/ml E-12 and 5 μg/ml 12-ol, namely, 50% difference in survival between PZA- and POA-treated infected cells in M. avium infection. As a reminder, one molecule of E-12 liberates one molecule of dodecanol and one of POA; thus, 10 μg of E-12 contains approximately 5 μg of the corresponding alcohol. However, for M. tuberculosis the results depicted in Fig. 3B (left) show a slightly higher activity of the ester relative to that of 12-ol or POA alone.
Cytotoxicity evaluation of the new prodrugs and alkanols to J774 cells.
To confirm that the decrease in CFU after treatment with the esters and long-chain alkanols were not due to potential cytotoxicity against J774 cells, causing the loss of infected macrophages in the culture, the alamarBlue test was used as described in Materials and Methods. The alamarBlue reagent allows the quantification of cell viability by measuring their metabolic functions. We verified that after 5 days of exposure, concentrations of prodrugs or n-alkanols equal to or below their MICs resulted in no reduction of macrophage viability in noninfected control host cells (Fig. 4). Cytotoxicity was tested in parallel for infected J774 cells for 7 days, but no effects were detected even at concentrations 10-fold higher than the MIC (not shown).
FIG 4.
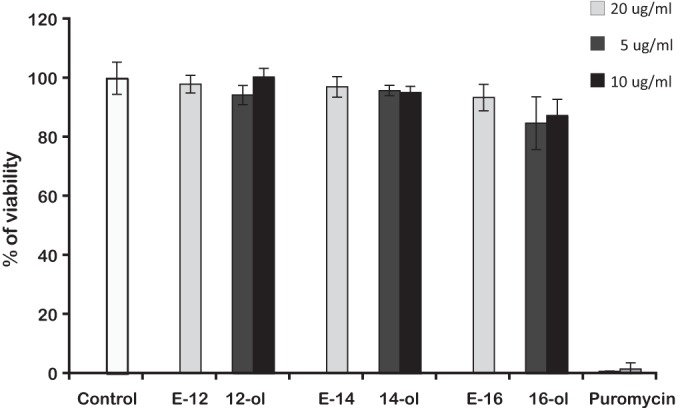
Cytotoxicity of PZA esters and the corresponding long-chain alkanols on host macrophages. The viability of macrophages is expressed as a percentage of survival relative to that of control macrophages.
DISCUSSION
The present study has shown that POA esters are more effective in clearing intracellular mycobacteria within J774 macrophages than PZA or POA (Fig. 2A and B). It was found that 100 μg/ml was an appropriate concentration to distinguish between susceptible strains (used as controls) and PZA-resistant M. tuberculosis. Moreover, MICs higher than 1,000 μg/ml were found for all mycobacteria that had natural or acquired resistance to PZA.
The POA esters, when tested against mycobacteria with natural or acquired resistance, were shown to be active, with MICs ranging from 5 to 80 μg/ml. This was true for five drug-resistant M. tuberculosis isolates and for M. bovis, including BCG. These are all mycobacteria whose PZA resistance mechanism is related to mutations in the pncA gene together with poor efflux pump mechanisms characteristic of this group of microorganisms.
It is intriguing that with M. avium, a naturally PZA-resistant species, both strains tested were highly susceptible to all esters but resistant to POA. Cynamon et al. also reported that POA esters, when obtained with similar alkanols (10-ol and 15-ol), presented activity against M. avium (12).
Since tested POA did not show activity against M. avium (Table 1), the activity presented by pyrazinoate esters could be due to increased accumulation of POA inside the cells, intrinsic activity of the ester molecules, or intrinsic activity of the alcohol moiety.
Regarding the first hypothesis, it is possible that POA is not active against M. avium because it cannot penetrate inside the bacteria; thus, the ester furnishes a way of delivering POA inside the cell; however, it is likely that M. avium possesses an effective POA efflux mechanism (11). If so, POA also should be expelled from the mycobacteria after being formed by hydrolysis, making the accumulation hypothesis less likely (unless the rate of entrance supplants the rate of efflux).
Two possibilities remain: either the esters present antimycobacterial activity per se or the alkanols have activity against M. avium. Since pyrazinoic acid esters are easily hydrolyzed to the corresponding alcohols in M. avium homogenate (Fig. 1), we favor the long-chain alcohols as the most likely agents accounting for the observed antimycobacterial activity. Indeed, it can be seen from Fig. 3B that the activity of the alkanol is comparable to the activity of the ester. This is not surprising, as the activity of the other moiety (POA) against M. avium is very low. It is known that Tween 80, a detergent used to disperse clumps of mycobacteria in liquid media cultures, can kill mycobacteria at acidic pH. Tween 80 releases oleic acid (23), and its structure has some similarities to long-chain alkanols. Both have a long hydrocarbon chain with a polar group capable of hydrogen bonding. Moreover, it is well known that alcohols can easily be oxidized in vivo to carboxylic acids (24, 25). Indeed, lethal effects of oleic acid and other fatty acids against mycobacteria were reported (26, 27). Other reports point to the lethal effect on M. tuberculosis of extracts of some South African plants enriched with palmitic, oleic, and linoleic acid and used to treat tuberculosis (28). More recently, the antimycobacterial activities of C6 to C13 n-alkanols were examined against M. smegmatis and M. tuberculosis H37Rv, with the best antimycobacterial activity being found with 10-ol (29), very similar to our 12-ol, for which we obtained the highest activity. The alcohols 12-ol, 14-ol, and 16-ol have logP values of 4.2, 5.2, and 7.2, respectively (as calculated by ALOGPS) (30). It seems possible that 14-ol and 16-ol, which are poorly soluble in water, do not dissolve satisfactorily in the test medium, explaining why better results are observed with alkanols of lower logP (4.2).
For M. tuberculosis, our results indicate that resistance was overcome by the release of POA and by the antimycobacterial effect of the long-chain alcohol released following POA ester hydrolysis (Fig. 3B, left), while for M. avium it is more likely that only the 12-ol contributed to the antimycobacterial effect (Fig. 3B, right). In a context of emergency with levels of MDR-TB increasing the world over, these preliminary results should provide a potential therapeutic alternative when other treatments have failed. This approach, applied to other active moieties, may lead to candidate dual drugs with enhanced activity against both PZA-susceptible and PZA-resistant isolates suitable for clinical development.
ACKNOWLEDGMENTS
We acknowledge Isabel Portugal (Faculty of Pharmacy, University of Lisbon) for providing the PZA-resistant M. tuberculosis strains. Support from the Portuguese Funding Agency, Fundação para a Ciência e Tecnologia (FCT), projects PTDC/SAU-FCF/101950/2008, PTDC/BIA-BCM/102123/2008, PTDC/SAU-MII/098024/2008, PIC/82859/2007, and PEst-OE/SAU/UI0775/2011, is gratefully acknowledged. D.P., M.F.S., and N.C. are Ph.D. students with FCT fellowships.
We are thankful to the NIBSC Centre for AIDS Reagents and BEI Resources for the offer of reagents.
REFERENCES
- 1.World Health Organization. 2012. Global tuberculosis control 2012. WHO publication no. WHO/HTM/TB/2012.6. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 2.Zhang Y, Mitchison D. 2003. The curious characteristics of pyrazinamide: a review. Int J Tuberc Lung Dis 7:6–21. [PubMed] [Google Scholar]
- 3.Zhang Y, Scorpio A, Nikaido H, Sun Z. 1999. Role of acid pH and deficient efflux of pyrazinoic acid in unique susceptibility of Mycobacterium tuberculosis to pyrazinamide. J Bacteriol 181:2044–2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mitchison DA. 1985. The action of antituberculosis drugs in short-course chemotherapy. Tubercle 66:219–225. doi: 10.1016/0041-3879(85)90040-6. [DOI] [PubMed] [Google Scholar]
- 5.Heifets LB, Iseman MD, Crowle AJ, Lindholm-Levy PJ. 1986. Pyrazinamide is not active in vitro against Mycobacterium avium complex. Am Rev Respir Dis 134:1287–1288. [DOI] [PubMed] [Google Scholar]
- 6.Grosset JH. 1992. Treatment of tuberculosis in HIV infection. Tuber Lung Dis 73:378–383. doi: 10.1016/0962-8479(92)90044-K. [DOI] [PubMed] [Google Scholar]
- 7.Cynamon MH, Klemens SP, Chou TS, Gimi RH, Welch JT. 1992. Antimycobacterial activity of a series of pyrazinoic acid esters. J Med Chem 35:1212–1215. doi: 10.1021/jm00085a007. [DOI] [PubMed] [Google Scholar]
- 8.Scorpio A, Zhang Y. 1996. Mutations in pncA, a gene encoding pyrazinamidase/nicotinamidase, cause resistance to the antituberculous drug pyrazinamide in tubercle bacillus. Nat Med 2:662–667. doi: 10.1038/nm0696-662. [DOI] [PubMed] [Google Scholar]
- 9.Scorpio A, Lindholm-Levy P, Heifets L, Gilman R, Siddiqi S, Cynamon M, Zhang Y. 1997. Characterization of pncA mutations in pyrazinamide-resistant Mycobacterium tuberculosis. Antimicrob Agents Chemother 41:540–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takiff HE, Cimino M, Musso MC, Weisbrod T, Martinez R, Delgado MB, Salazar L, Bloom BR, Jacobs WR Jr. 1996. Efflux pump of the proton antiporter family confers low-level fluoroquinolone resistance in Mycobacterium smegmatis. Proc Natl Acad Sci U S A 93:362–366. doi: 10.1073/pnas.93.1.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raynaud C, Laneelle MA, Senaratne RH, Draper P, Laneelle G, Daffe M. 1999. Mechanisms of pyrazinamide resistance in mycobacteria: importance of lack of uptake in addition to lack of pyrazinamidase activity. Microbiology 145(Part 6):1359–1367. doi: 10.1099/13500872-145-6-1359. [DOI] [PubMed] [Google Scholar]
- 12.Cynamon MH, Gimi R, Gyenes F, Sharpe CA, Bergmann KE, Han HJ, Gregor LB, Rapolu R, Luciano G, Welch JT. 1995. Pyrazinoic acid esters with broad spectrum in vitro antimycobacterial activity. J Med Chem 38:3902–3907. doi: 10.1021/jm00020a003. [DOI] [PubMed] [Google Scholar]
- 13.Bergmann KE, Cynamon MH, Welch JT. 1996. Quantitative structure-activity relationships for the in vitro antimycobacterial activity of pyrazinoic acid esters. J Med Chem 39:3394–3400. doi: 10.1021/jm950538t. [DOI] [PubMed] [Google Scholar]
- 14.Simoes MF, Valente E, Gomez MJ, Anes E, Constantino L. 2009. Lipophilic pyrazinoic acid amide and ester prodrugs stability, activation and activity against M. tuberculosis. Eur J Pharm Sci 37:257–263. doi: 10.1016/j.ejps.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 15.Jordao L, Bleck CK, Mayorga L, Griffiths G, Anes E. 2008. On the killing of mycobacteria by macrophages. Cell Microbiol 10:529–548. [DOI] [PubMed] [Google Scholar]
- 16.Portugal I, Barreiro L, Moniz-Pereira J, Brum L. 2004. pncA mutations in pyrazinamide-resistant Mycobacterium tuberculosis isolates in Portugal. Antimicrob Agents Chemother 48:2736–2738. doi: 10.1128/AAC.48.7.2736-2738.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anes E, Kuhnel MP, Bos E, Moniz-Pereira J, Habermann A, Griffiths G. 2003. Selected lipids activate phagosome actin assembly and maturation resulting in killing of pathogenic mycobacteria. Nat Cell Biol 5:793–802. doi: 10.1038/ncb1036. [DOI] [PubMed] [Google Scholar]
- 18.Valente E, Simoes MF, Testa B, Constantino L. 2011. Development of a method to investigate the hydrolysis of xenobiotic esters by a Mycobacterium smegmatis homogenate. J Microbiol Methods 85:98–102. doi: 10.1016/j.mimet.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 19.Sethi S, Mandal J, Kumar P, Kumar S, Meharwal S, Jindal SK, Sharma M. 2007. Susceptibility testing of Mycobacterium tuberculosis by broth microdilution method: a rapid alternative method. Diagn Microbiol Infect Dis 57:447–449. doi: 10.1016/j.diagmicrobio.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 20.Butler WR, Kilburn JO. 1982. Improved method for testing susceptibility of Mycobacterium tuberculosis to pyrazinamide. J Clin Microbiol 16:1106–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heifets LB, Iseman MD. 1985. Radiometric method for testing susceptibility of mycobacteria to pyrazinamide in 7H12 broth. J Clin Microbiol 21:200–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kushner S, Bach DH Jr, Centola FLD, Sanjurjo JL, Williams JH. 1955. Experimental chemotherapy of tuberculosis. III. Ethyl mercaptan and related compounds in tuberculosis. J Am Chem Soc 77:1152–1155. [Google Scholar]
- 23.Dubos RJ, Davis BD. 1946. Factors affecting the growth of tubercle bacilli in liquid media. J Exp Med 83:409–423. doi: 10.1084/jem.83.5.409. [DOI] [PubMed] [Google Scholar]
- 24.Testa B, Kramer SD. 2007. The biochemistry of drug metabolism–an introduction. Part 2. Redox reactions and their enzymes. Chem Biodivers 4:257–405. [DOI] [PubMed] [Google Scholar]
- 25.Testa B, Kramer SD. 2007. The biochemistry of drug metabolism–an introduction: part 3. Reactions of hydrolysis and their enzymes. Chem Biodivers 4:2031–2122. [DOI] [PubMed] [Google Scholar]
- 26.Kanetsuna F. 1985. Bactericidal effect of fatty acids on mycobacteria, with particular reference to the suggested mechanism of intracellular killing. Microbiol Immunol 29:127–141. doi: 10.1111/j.1348-0421.1985.tb00811.x. [DOI] [PubMed] [Google Scholar]
- 27.Stavri M, Schneider R, O'Donnell G, Lechner D, Bucar F, Gibbons S. 2004. The antimycobacterial components of hops (Humulus lupulus) and their dereplication. Phytother Res 18:774–776. doi: 10.1002/ptr.1527. [DOI] [PubMed] [Google Scholar]
- 28.Seidel V, Taylor PW. 2004. In vitro activity of extracts and constituents of Pelagonium against rapidly growing mycobacteria. Int J Antimicrob Agents 23:613–619. doi: 10.1016/j.ijantimicag.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 29.Mukherjee K, Tribedi P, Mukhopadhyay B, Sil AK. 2013. Antibacterial activity of long-chain fatty alcohols against mycobacteria. FEMS Microbiol Lett 338:177–183. doi: 10.1111/1574-6968.12043. [DOI] [PubMed] [Google Scholar]
- 30.Tetko IV. 2005. Computing chemistry on the web. Drug Discov Today 10:1497–1500. doi: 10.1016/S1359-6446(05)03584-1. [DOI] [PubMed] [Google Scholar]