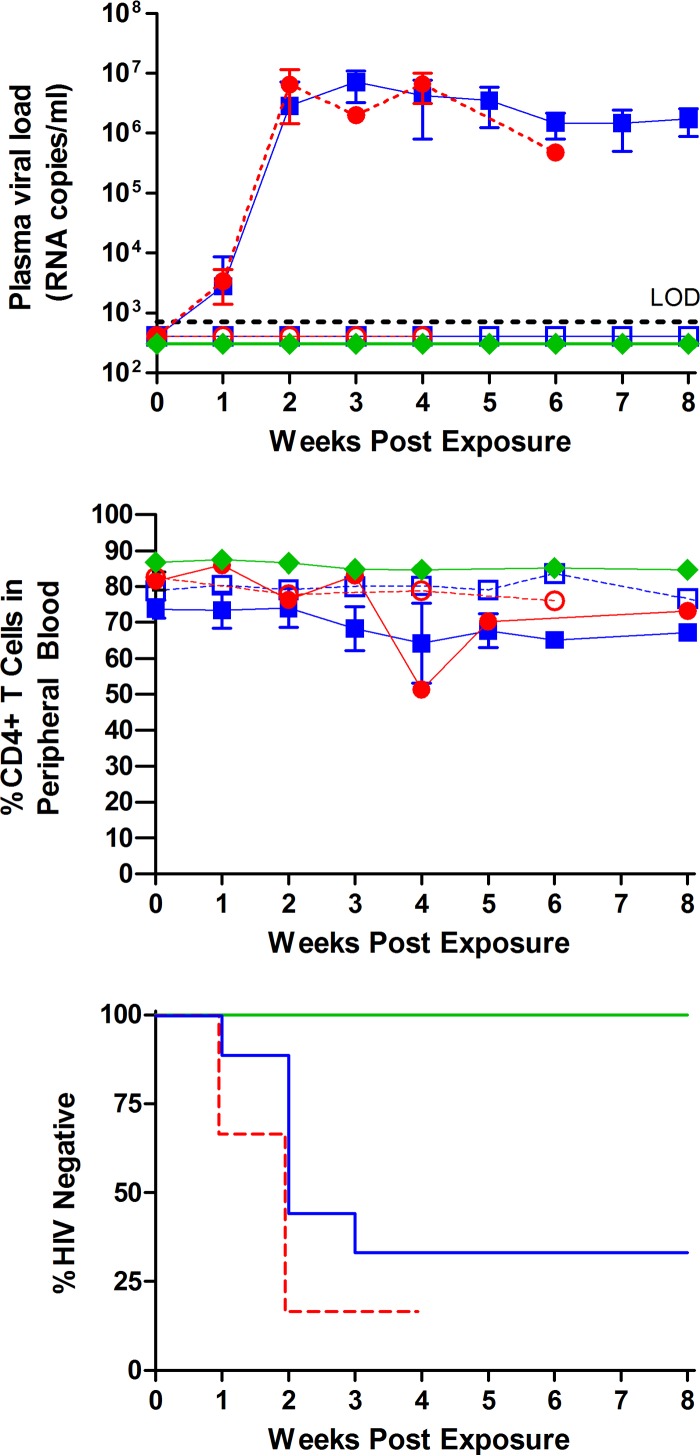
FIG 1.
Analysis of the effect of semen on vaginal cell-free HIV-1 transmission and on the protective effect of topically administered maraviroc. Symbols in the top and middle panels: open blue squares, semen, infection negative (n = 3); solid blue squares, semen, infection positive (n = 6); open red circles, vehicle, infection negative (n = 1); solid red circles, vehicle, infection positive (n = 5); green diamonds, top panel, maraviroc plus semen (n = 6), and bottom panel, maraviroc treated (n = 6). BLT humanized mice were exposed vaginally to HIV-1JR-CSF (7.0 × 105 TCIU) in the presence (n = 9) or absence (n = 6) of human semen and in the presence of semen and maraviroc (5 mM). (Top panel) Infection was determined based on the presence or absence of viral RNA in plasma over the course of the experiment. Transmission in the vehicle-treated mice was 5/6, in the mice receiving virus resuspended in semen, 6/9 were positive, and in the mice receiving virus resuspended in semen after maraviroc administration, 0/6 were positive for viral RNA. LOD, limit of detection. (Middle panel) Longitudinal analysis of peripheral blood CD4+ T cell levels of infected and noninfected mice. Symbols in the bottom panel: solid green line, maraviroc plus semen (n = 6); solid blue line, semen (n = 9); dashed red line, vehicle (n = 6). Statistical analysis was performed using the Mantel-Cox log-rank test. Over the course of the experiment, all six maraviroc-treated mice remained HIV negative (P = 0.01). The indicated error bars represent the standard deviations.