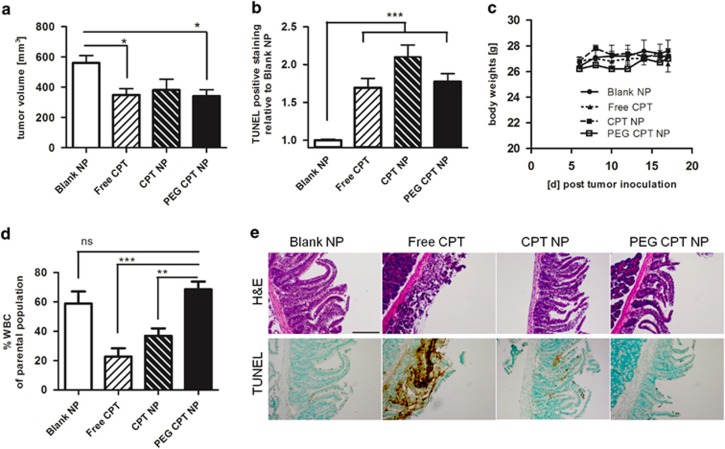
Figure 2.
Growth inhibition of syngeneic colorectal tumors and reduction of chemotherapy-associated adverse effects through CPT nanoencapsulation. (a) MC38 colorectal tumor volume on day 17 after cell inoculation in C57BL/6 mice and serial treatment with CPT (2.5 mg/kg) on day 6, 10 and 14, mean±S.E.M., n≥8. (b) Quantification of TUNEL-positive cells analyzed in isolated tumors, mean±S.E.M., n=3. (c) Mouse body weight during the treatment regime, mean±S.E.M., n≥8. (d) Quantification of circulating white blood cells (WBC) analyzed by flow cytometry as %WBC of parental population, mean±S.E.M., n≥8. (e) Images of paraffin-embedded sections of the jejunum stained with H&E or TUNEL after serial CPT treatment, representative scale bar equals 100 μm