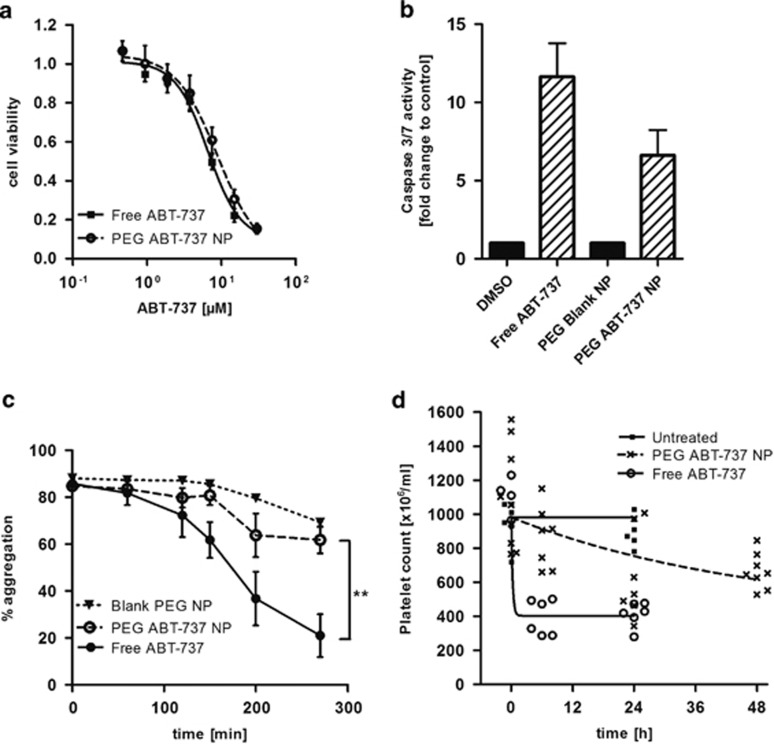
Figure 3.
Nanoencapsulation of ABT-737 inhibits thrombocytopenic effects. (a) Dose–response curves of HCT116 cells after treatment with free or PEG-PLGA encapsulated ABT-737 for 48 h assessed by MTT cell viability assay, mean±S.E.M., n=3. (b) Caspase 3/7 activity in HCT116 cells after treatment with free and nanoencapsulated ABT-737 (5 μM) for 6 h, relative to control treated cells, mean±S.E.M., n=4. (c) Aggregometry of washed human platelets after pre-incubation with free or nanoencapsulated ABT-737 (1 μM), platelet aggregation was induced by U46619 (2 μM), mean±S.E.M., n=4. (d) Time course of platelet counts in C57BL/6 mice following intraperitoneal injection of 50 mg/kg of free ABT-737 or ABT-737 NPs, n≥5. A significant difference was observed between the two ABT-737 treatment groups (P<0.0001).The best fit model (highest Schwarz Bayesian Criteria=−398.2) indicated a difference in the rate of change by ~177-fold