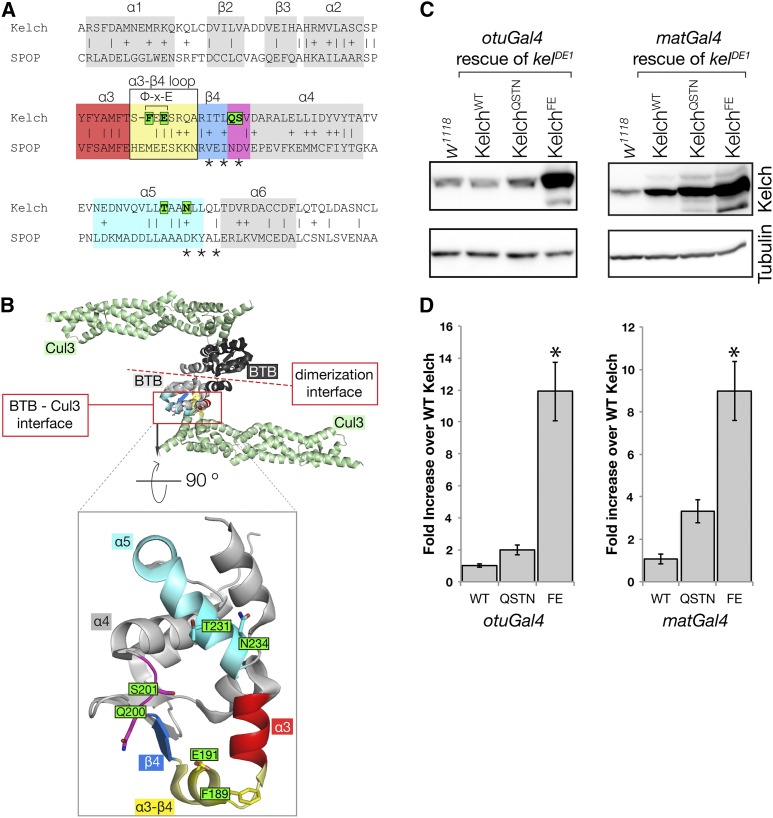
Figure 4.
Mutagenesis of the Kelch BTB domain to disrupt Kelch–Cul3 binding. (A) Alignment of Kelch with the sequence SPOP, a BTB domain whose structure has been solved in complex with the Cul3 N-terminal domain. α-helix and β-sheet numbering are as in Errington et al. (2012). Residues mutated in this study are boxed in green; asterisks mark positions of residues mutated previously (Xu et al. 2003) to disrupt the BTB–Cul3 association. The Φ-X-E motif contributes significantly to BTB–Cul3 binding, as the hydrophobic residue inserts into a hydrophobic pocket in Cul3 and the glutamate residue forms a hydrogen bond with a Cul3 α-helix. Sequences highlighted by colored boxes correspond to similarly colored structural elements in B. (B) Structure of the SPOP BTB domain in complex with Cul3 (PDB 4EOZ; Errington et al. 2012). The structure is a heterotetramer consisting of two Cul3 N-terminal domains (green) and two BTB domains (black and gray). Two Cul3 α-helices make extensive contacts with one face of the BTB domain. Boxed region shows isolated view of Cul3 binding surface on the SPOP BTB domain. Kelch residues targeted in this study were generally not conserved in SPOP and were modeled into this representation using MacPyMol. The amino acid sequence positions targeted for mutagenesis are oriented toward Cul3, and each was found to contribute to the interaction surface in the solved structures (Errington et al. 2012; Canning et al. 2013; Ji and Prive 2013). (C) Western analysis of ovaries from kelDE1 females rescued with Kelch containing the indicated mutations in the BTB domain. (D) Mean protein expression levels measured relative to endogenous w1118 control. The expression level from the KelchWT cDNA rescue in each experiment was set at 1. Asterisk indicates statistically significant difference compared to expression from KelchWT cDNA (one-way ANOVA, Dunnett multiple comparison test, P < 0.001). Error bars indicate the standard error of the mean. QSTN, Q200A S201A T232A N234A; FE, F189E E191A.