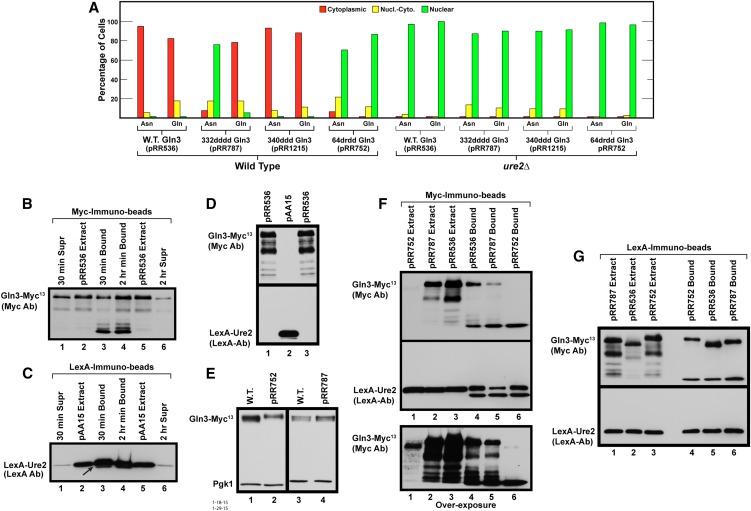
Figure 7.
Ure2 binds to and functions with mutant forms of Gln3-Myc13. (A) The effects of deleting URE2 on glutamine- or asparagine-grown wild-type and mutant Gln3L332D,L336D,M340D,L343D-Myc13 (pRR787), Gln3M340D,L343D,L345D-Myc13 (pRR1215), or Gln3L64D,L67R,L71D,F73D-Myc13 (pRR752) localization. Wild-type (pRR536) or mutant (pRR787, pRR1215, and pRR752) plasmids were transformed into wild-type (JK9-3da) or ure2Δ (RR215) strains. These transformants were assayed and the data presented as described in Figure 1, C–E. (B and C) Time-dependent binding of wild-type (pRR536) Gln3-Myc13 and NLS-LexA-Ure2 to anti-Myc and anti-LexA immunobeads. An extract of TB50 transformed with both pRR536 and pAA15 was bound to anti-Myc or anti-LexA immunobeads, respectively, for either 30 min or 2 hr at 4°. Supernatants (Supr) remaining after proteins in the extracts were bound to the immunobeads and proteins eluted from the immunobeads (Bound) and extracts from wild-type (pRR536) or NLS-LexA-Ure2 (pAA15) cultures were subjected to Western blot analyses. The blots were probed with either anti-Myc (B) or anti-LexA (C) antibodies. (D) Anti-Myc and anti-LexA antibodies are highly specific, exhibiting no cross-reaction. Extracts were prepared from glutamine-grown transformants containing either wild-type Gln3-Myc13 (pR536) or NLS-LexA-Ure2 (pAA15), as described in Materials and Methods. These extracts were then loaded into parallel gels, and the blots emanating from them were probed with anti-Myc (top blot) or anti-LexA antibodies (bottom blot). (E) Wild-type Gln3-Myc13 (pRR536), Gln3L64D,L67R,L71D,F73D-Myc13 (pRR752), and Gln3L332D,L336D,M340D,L343D-Myc13 (pRR787) proteins are present in similar amounts in vivo. Extracts from either wild-type (pRR536) or mutant (pRR752 and pRR787) Gln3-Myc13 YNB-asparagine-grown cultures were precipitated with trichloroacetic acid and subjected to SDS-PAGE, and the blots were simultaneously probed with anti-Myc and anti-Pgk1 antibodies. Pgk1 was used to assess gel loading and transfer. Compare them with the behavior of extracts analyzed in B, F, and G. (F) NLS-LexA-Ure2 immunoprecipitates with Gln3-Myc13. Extracts were prepared from an asparagine-grown wild-type recipient TB50 transformed with pAA15 (NLS-LexA-Ure2) and either pRR536 (wild-type Gln3-Myc13), pRR787 (Gln3L332D,L336D,M340D,L343D-Myc13), or pRR752 (Gln3L64D,L67R,L71D,F73D-Myc13). The extracts were bound to anti-Myc immunobeads for 30 min at 4° to immunoprecipitate Gln3-Myc13 and proteins potentially bound to it. Extracts and proteins eluted from the anti-Myc immunobeads then were subjected to two parallel Western blot analyses. One of the blots was probed with anti-Myc antibodies (top and bottom blots) and the other with anti-LexA antibodies (middle blot). The blot probed with anti-Myc antibodies (top blot) was vastly overexposed (bottom blot) to visualize the degraded Gln3-Myc13 proteins it contained. (G) Gln3-Myc13 immunoprecipitates with NLS-LexA-Ure2. Extracts were prepared from a glutamine-grown wild-type recipient TB50 transformed with pAA15 (NLS-LexA-Ure2) and pRR536 (wild-type Gln3-Myc13), pRR787 (Gln3L332D,L336D,M340D,L343D-Myc13), or pRR752 (Gln3L64D,L67R,L71D,F73D-Myc13). The extracts were bound to anti-LexA immunobeads for 15 min at 4° to immunoprecipitate NLS-LexA-Ure2 and proteins potentially bound to it. Extracts and proteins eluted from the anti-LexA immunobeads then were subjected to two parallel Western blot analyses. One of the blots then was probed with anti-Myc antibodies (top blot) and the other with anti-LexA antibodies (bottom blot).