Figure 1. Modeled structures of GRPR-targeted ProCAs and in silico docking of GRPR-targeted ProCAs to GRPR.
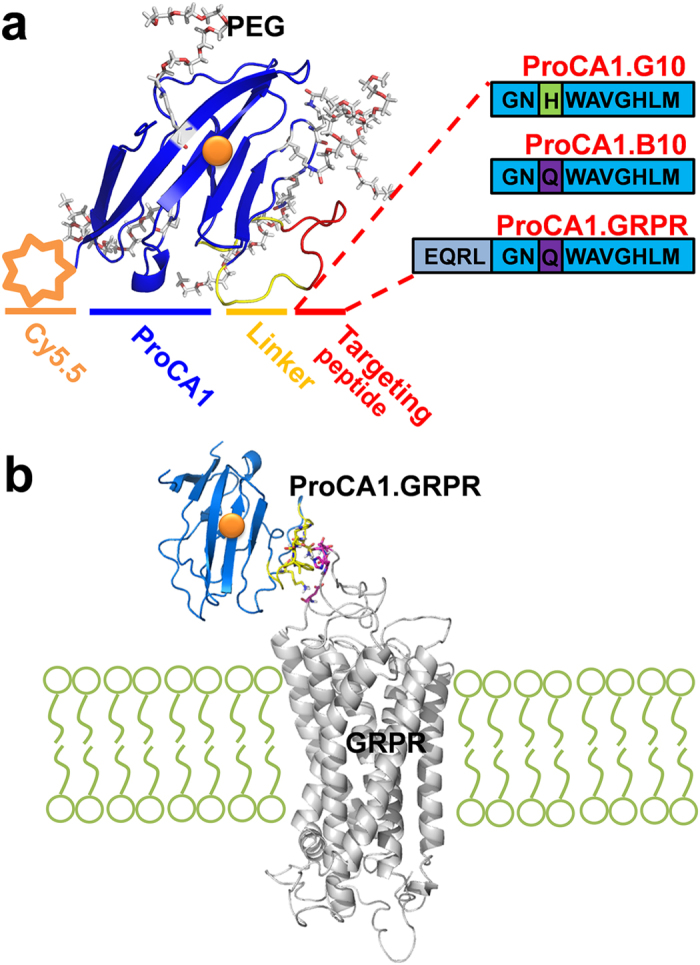
(a) Optimizing GRPR targeting peptide for molecular imaging by MRI. The modeled structure of ProCA1 variants (blue) with grafted GRPR targeting peptide from GRP or bombesin (red). ProCA1 is a protein-based MRI contrast agent derived from domain 1 of rat CD2 with several mutations to form a gadolinium (orange) binding pocket on its surface. ProCA1.B10 and ProCA1.G10 contain 10 amino acids at the C-terminal of bombesin and GRP, respectively. They share the same peptide sequence except one residue (H/Q) difference which is related to the GRP/bombesin-GRPR binding affinity. ProCA1.GRPR contains the whole sequence of bombesin with 14 amino acids. The ProCA1 and GRPR targeting peptide were connected through a flexible linker (yellow). These GRPR-targeted contrast agents are also PEGylated and conjugated with Cy5.5. (b) In silico docking of GRPR-targeted ProCAs to GRPR by HADDOCK.
