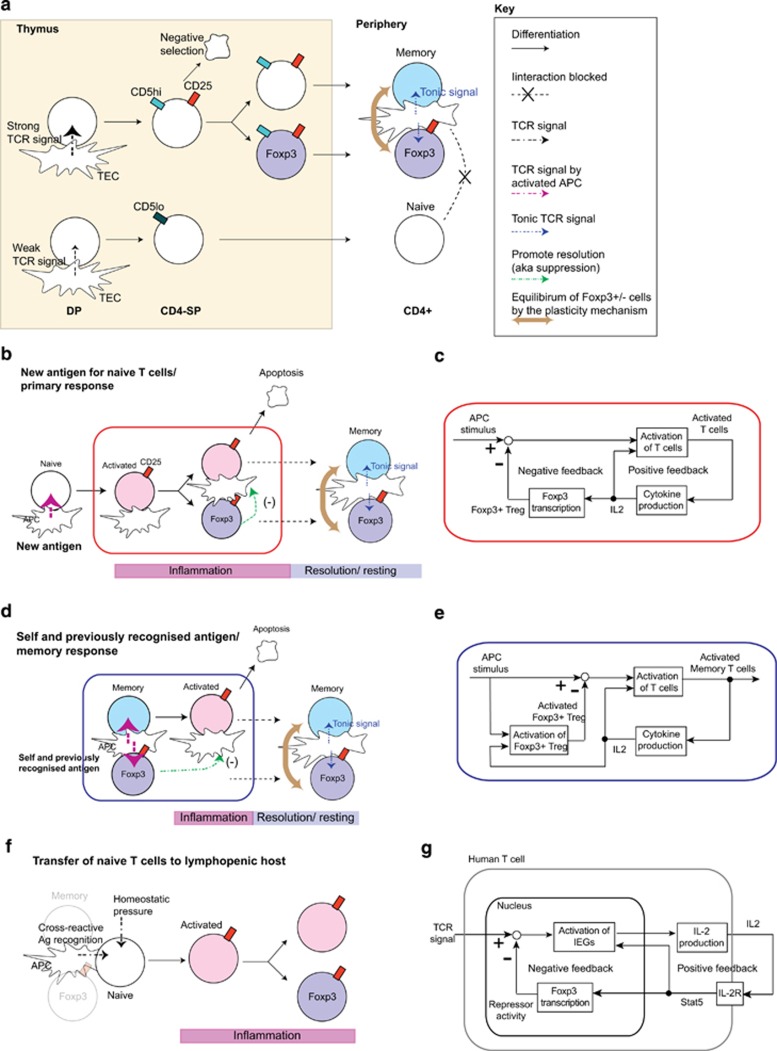
Figure 1.
Feedback control perspective of T-cell regulation. (a) The proposed model for the T-cell regulation is depicted. Self-reactive thymic CD4-SP receive strong TCR signals from antigen/MHC complex on thymic epithelial cells (TEC) and other APCs. These cells may die by negative selection or survive by expressing CD25 and upregulating the expression of CD5 (CD5high), which is a negative regulator of TCR signaling. Some CD25+ CD4-SP express Foxp3 and become Foxp3+ Tregs in the periphery, while some of the others, we argue, become Foxp3− memory-like T cells (memory). On the other hand, thymic T cells with less self-reactive TCRs may receive weak TCR signals only, remain CD5low, do not express CD25 and become naive T cells in the periphery. Thus both Tregs and memory-like T cells are more self-reactive than naive T cells and therefore interact more frequently with APCs that present the same or similar self-antigens in the periphery, receiving tonic TCR signaling, and protecting the antigenic niche from naive T cells. (b) Upon encountering with a totally new antigen to the immune system, only some of the naive T cells can respond to the antigen and become CD25+ activated T cells. Many of these activated T cells die by apoptosis (that is, activation-induced cell death), but some differentiate into Tregs, promoting the resolution of the response (negative feedback control), and others may become memory T cells, after the resolution. Thus a new antigenic niche is created and occupied by both Tregs and memory T cells, which are maintained by tonic TCR signal in the same manner as for thymus-derived, self-reactive T cells. (c) Control mechanism of naive T cells to new antigens shown in panel (b). (d) Upon encountering with self-antigen or similar antigen on activated APC, both memory(-like) T cells and Tregs immediately respond and are activated. Thus the response will be rapid but be resolved earlier because of the negative feedback by the preexisting Tregs. (e) Control mechanism of memory-like T cells and Tregs to self-antigens or experienced antigens shown in panel (d). (f) Various ‘Treg-depletion' experiments in fact provide to naive T cells an access to all antigenic niches. This effect is most dramatic when both Tregs and memory-like T cells are depleted, and naive T cells have the full access to all niches. (g) Possible mechanism for the control of T-cell activation by transient FOXP3 expression in human activated T cells. TCR signal induces and activates immediate early genes (IEGs), which transcribe the IL2 gene. IL-2 protein is secreted and received by those activated T cells in an autocrine manner. IL-2 signal via IL-2R (including CD25) activates STAT5, which positively regulate the activation of IEGs and FOXP3 transcription. FOXP3 represses IEGs by physically interacting with them or repressing their transcription.