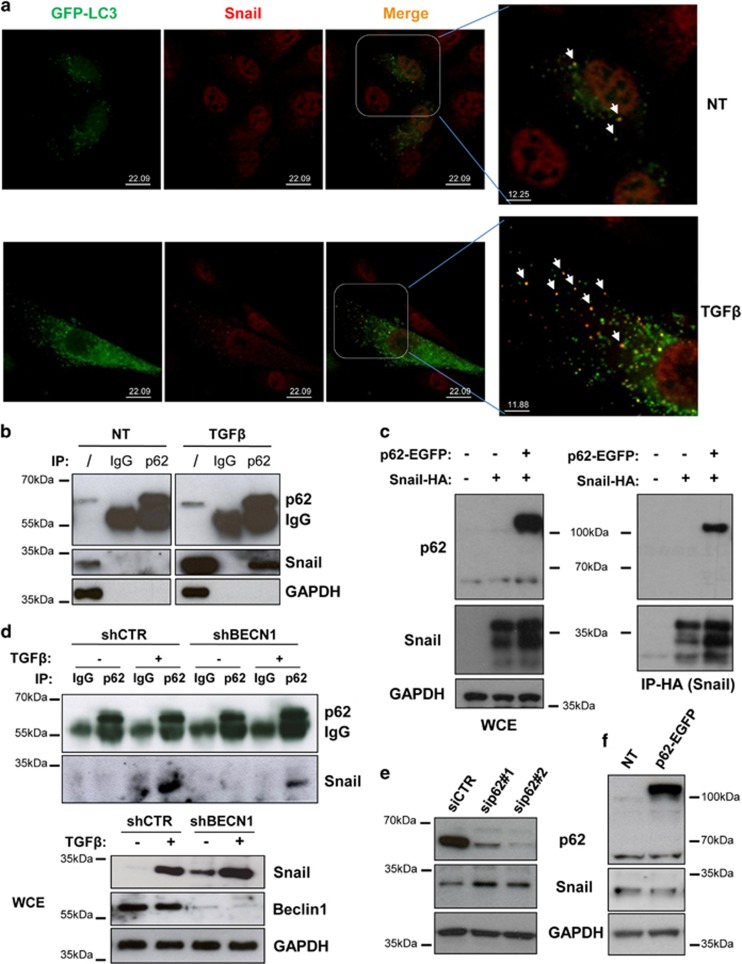
Figure 3.
Autophagy degrades Snail in hepatocytes through the action of p62. (a) Colocalisation of LC3 with Snail was analysed by immunofluorescence staining for Snail (red) in hepatocytes expressing GFP-LC3 (green). Cells, either untreated or stimulated with TGFβ, were treated with BafA1 (5 ng/ml) for 3 h, fixed, permeabilised, stained with an anti-Snail antibody and analysed by confocal microscopy. In the magnified panels, arrows indicate the colocalisation of GFP-LC3 with Snail. (b) Immunoblotting analysis for Snail and p62 following immunoprecipitation of either p62 or using an isotype-matched control mAb from lysates of parental cells either untreated or stimulated with TGFβ (2 ng/ml) for 3 h. GAPDH (glyceraldehyde 3-phosphate dehydrogenase) was used for protein loading control. (c) Immunoblotting analysis for Snail and p62 following immunoprecipitation for HA (Snail) in parental cells or in hepatocytes overexpressing Snail-HA either in the presence or absence of p62-EGFP (48 h posttransfection). GAPDH was used for protein loading control. (d) Immunoblotting analysis of Snail and p62 following immunoprecipitation of either p62 or using an isotype-matched control mAb from lysates of autophagy-deficient (shBECN1) or control (shCTR) cells either untreated or stimulated with TGFβ (2 ng/ml) for 3 h. In whole-cell extracts (bottom panels), BECN1 and GAPDH were used for analysing the BECN1-silencing levels and as protein loading control, respectively. (e and f) Immunoblotting analysis for Snail and p62 of hepatocytes in which the expression of p62 was either inhibited by two specific siRNAs (e) or increased by transfecting a p62-EGFP-expressing vector (f). GAPDH levels were used for protein loading control