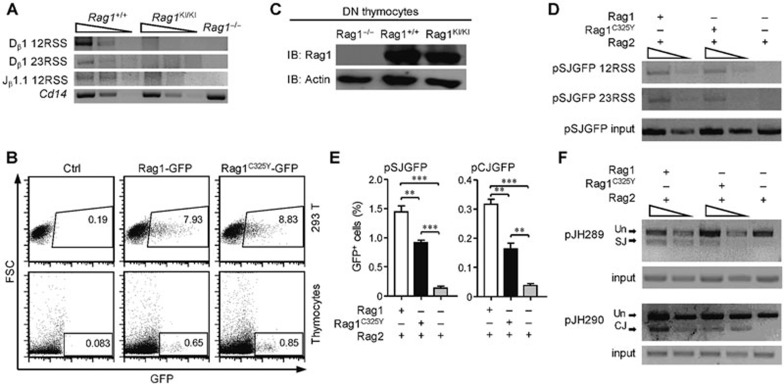
Figure 3.
RAG1C325Y is unable to catalyze chromosomal substrates. (A) V(D)J recombination stopped at the cleavage phase in RAG1KI/KI mice. Detection of the signal ends of 5′ Dβ1, 3′ Dβ1 and 5′ Jβ1.1 in DN3 thymocytes sorted from indicated mice by LM-PCR. Below, amplification of a Cd14 fragment (input control). PCR amplification was performed on serial fivefold dilutions of genomic DNA ligated to a BW-linker. (B) RAGC325Y exhibited a normal expression level compared to WT RAG1 in vitro. RAG1-GFP and RAGC325Y-GFP were transiently transfected into thymocytes and HEK-293T cells. After 12 h, GFP expression and intensity was analyzed by FACS. (C) RAGC325Y exhibited a normal expression level compared to WT RAG1 in vivo. Western blot analysis of RAG1 and RAG1C325Y expression in DN3 thymocytes sorted from indicated mice. β-actin expression was assessed as a loading control. (D) LM-PCR analysis of the signal end of pSJGFP generated in HEK-293T cells. PCR amplification was performed on serial fivefold dilutions of template DNA. Control PCR amplification of a non-rearranging locus on pSJGFP was performed to normalize the input DNA. SE, signal end. (E) Fluorescent reporter substrates recombination assay in HEK-293T cells. The GFP level was measured by flow cytometric analysis. The percentages of GFP+ cells are shown (means ± SD; n = 3; ***P < 0.001 and **P < 0.01 by Student's t-test). (F) The recombination products of pJH289 and pJH290 in HEK-293T were analyzed by PCR. Un, unrearranged; CJ, coding joint; SJ, signal joint. PCR amplification was performed on serial three-fold dilutions of template DNA. Control PCR amplification of a non-rearranging locus on pJH289 and pJH290 was performed to normalize the input DNA. All results are representative of three independent experiments.