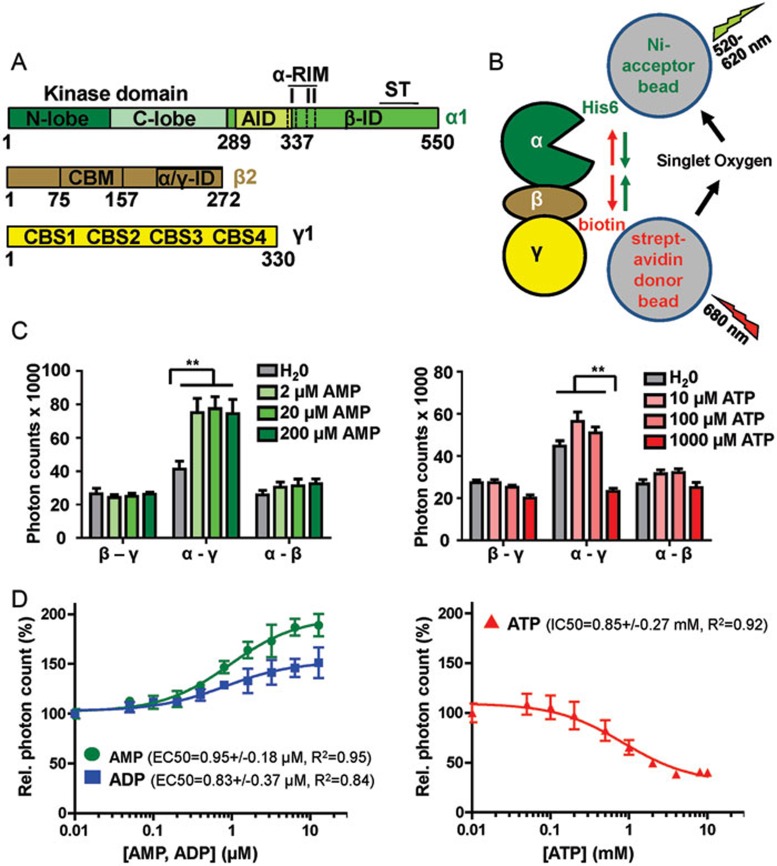
Figure 1.
AMP and ATP induce opposing conformational changes in AMPK. (A) Schematic presentation of the three subunits of the AMPK isoform α1β2γ1 (human AMPK has two alternative α-subunits, two β-subunits, and three γ-subunits that can form a total of 12 different isoforms). Amino acid positions are indicated below domain boundaries. AID, autoinhibitory domain; CBM, carbohydrate-binding module; CBS, cystathionine β-synthetase motif-type adenine nucleotide binding site; αRIM, regulatory subunit-interacting motif loop, which contains two motifs, αRIM-1 (I) and αRIM-2 (II); α/γ-ID, C-terminal α-subunit and β-subunit interaction domain; β-ID, C-terminal β-subunit interaction domain; ST, ST-loop. (B) Cartoon of AlphaScreen luminescence proximity assay for AMPK with a His6-tag at the N-terminus of the α-subunit and a biotin tag at the N-terminus of the γ-subunit. The tags bind to streptavidin donor and Ni-acceptor nanobeads, respectively, and bring donor- and acceptor-beads into close proximity. Donor beads contain a photosensitizer that upon activation at 680 nm converts ambient oxygen into singlet oxygen. With decreasing distance (green arrows), more short-lived (t1/2 = 4 μsec) singlet oxygen will diffuse to the acceptor beads to transfer energy from the singlet oxygen to thioxene derivatives in the acceptor beads, resulting in increased light emission at 520-620 nm. Since the energy transfer between beads is chemically mediated by singlet oxygen, signals are insensitive to bead orientation. (C) Adenine nucleotides modulate AlphaScreen signals of AMPK with selectively tagged subunits (β-γ: α1-biotin-β2-His6-γ1, α-γ: His6-α1-β2-biotin-γ1, α-β: His6-α1-biotin-β2-γ1; n = 3; error bars, SD). **P < 0.01 (Student's t-test). (D) Dose-response curves of the effect of adenine nucleotides on the AlphaScreen signal (n = 3; error bars, SD). Note that concentration of AMP and ADP are in μM and of ATP in mM.