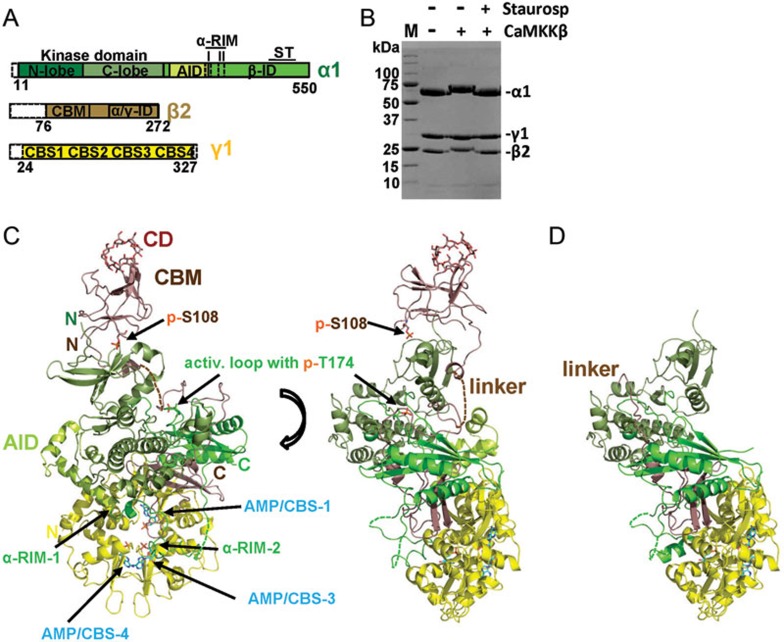
Figure 2.
Structure overview of human AMPK bound to AMP, cyclodextrin and staurosporine. (A) Schematic presentation of the crystallization construct, using the same color code as in the following structure panels. Unstructured termini omitted in the crystallization construct are shown as white dashed boxes. (B) Coomassie-stained SDS-PAGE gel of purified AMPK prior and after phosphorylation by CaMKKβ, in the presence and absence of the kinase inhibitor staurosporine. M: protein standards, with molecular weights indicated in kDa. (C) Structure overview of phosphorylated AMPK in two different orientations. (D) Structure overview of non-phosphorylated AMPK. AID, autoinhibitory domain; CBM, carbohydrate-binding module; CBS, cystathionine β-synthetase motif-type adenine nucleotide binding site; CD, cyclodextrin; αRIM, regulatory subunit-interacting motif; α/γ-ID, C-terminal α-subunit and β-subunit interaction domain; β-ID, C-terminal β-subunit interaction domain; ST, ST-loop.