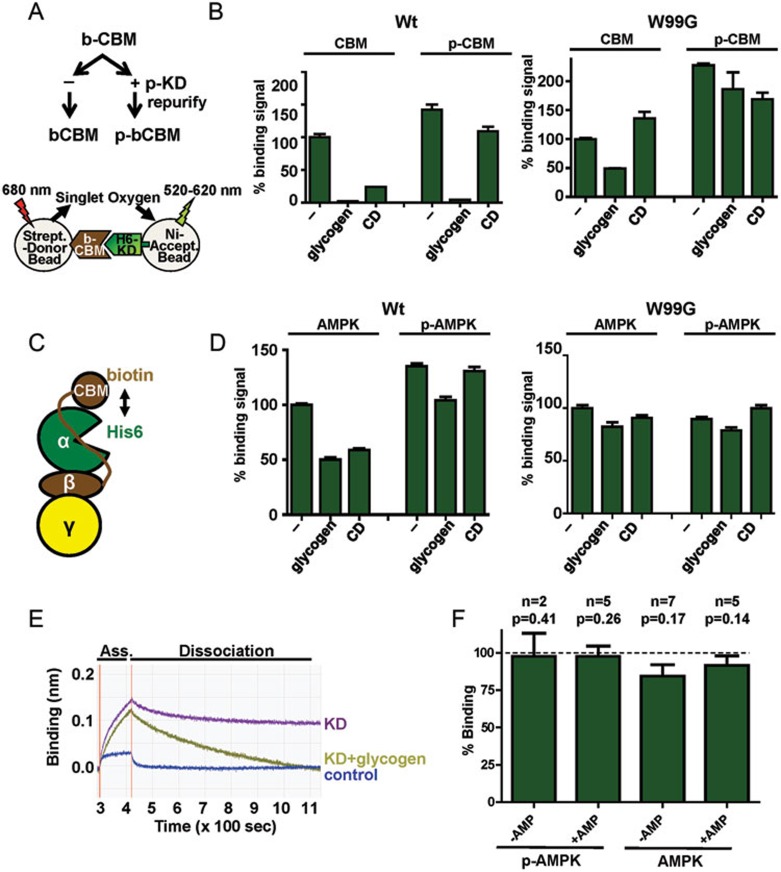
Figure 3.
Carbohydrates destabilize the CBM-KD interaction. (A) Experimental outline. b-, biotinylated, p-, phosphorylated. (B) AlphaScreen interaction between phosphorylated and non-phosphorylated wild-type (WT) and mutant (W99G) biotin-CBM and the isolated His6-KD in trans (n = 3; error bars, SD). (C) Cartoon presentation of the AlphaScreen experiment in the context of holo-AMPK. (D) Glycogen and cyclodextrin reduce the AlphaScreen signal for holo-AMPK containing biotinylated CBM and His6-tagged KD (n = 3; error bars, SD). (E) Interaction of the CBM with KD by biolayer interferometry. Biotinylated CBM was immobilized to the biosensor of an Octed Red instrument. Addition of the untagged KD in the presence or absence of glycogen resulted in an increase in mass due to CBM binding (association), followed by a decrease after washing away unbound protein due to dissociation of the interaction. (F) Effect of glycogen on the kinase activity of phosphorylated and non-phosphorylated AMPK in the presence and absence of 200 μM AMP. Activity was determined by radioactive kinase assays as shown in Supplementary information, Figure S1 and represented as % activity in the presence of glycogen relative to the absence of glycogen (error bars, SD; n, number of replicate experiments using independent AMPK preps; P, probability for observed inhibition occurring by chance (one-sided, paired t-test); glycogen, 1 mg/ml glycogen, CD, 2 mM cyclodextrin.