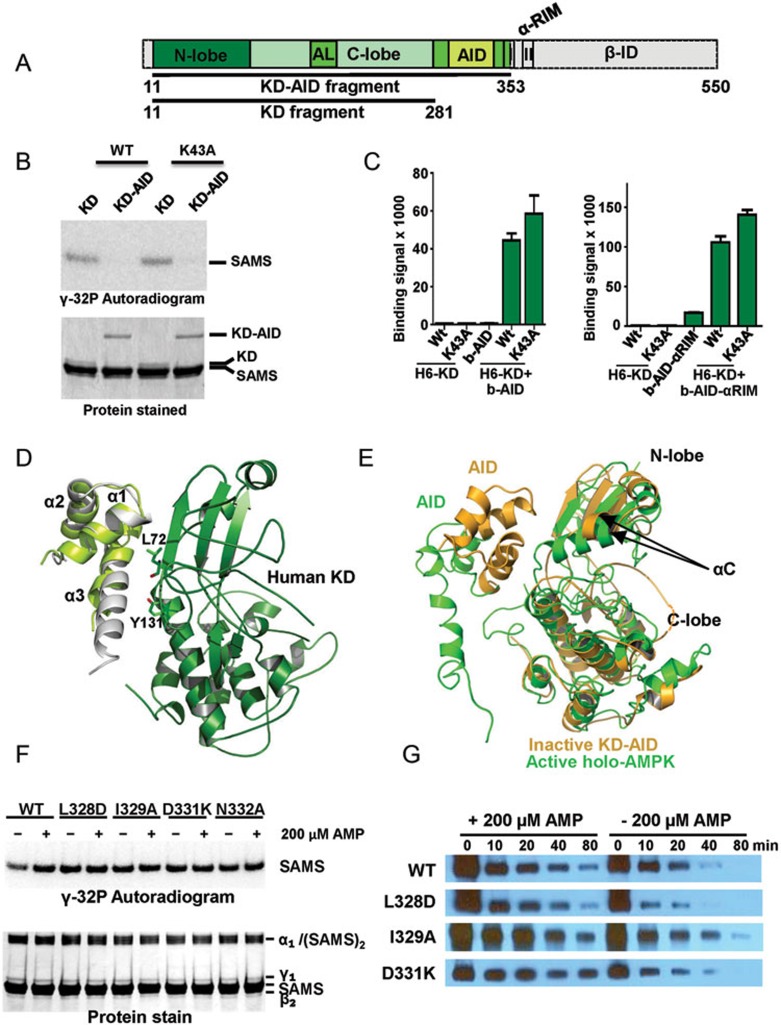
Figure 4.
The human AID can bind the KD hinge to keep the KD in an inactive, open conformation. (A) Schematic presentation of the human α1-subunit. Numbers indicate amino acid positions. The KD-AID fragment used for crystallization (α1 11-353) is shown in color. AL, activation loop; I, αRIM-1; II, αRIM-2. (B) The AID inhibits AMPK KD catalytic activity. Wild-type (WT) and K43A recombinant KD (α1 11-281) and KD-AID (α1 11-353) were incubated with His6GST-SAMS peptide substrate and [γ-32P]-ATP. Terminated reactions were separated by SDS-PAGE and subjected to autoradiography. (C) AlphaScreen interaction between wild-type (WT) and K43A His6-tagged KD and biotinylated AID (α1[282-353]), left) or AID-αRIM (α1[282-392]), right). (D) Structure model of the human inhibitory cis KD-AID complex. The three α-helices of the human AID are shown in light green, overlaid on the structure of the yeast AID (PDB, 3H4J) in grey. Key AID-interacting residues of the KD are shown in stick presentation. (E) Superposition of the KD-AID inhibitory complex from D (pale orange) with the KD-AID conformation in the context of activated holo-AMPK (green; detail from Figure 2D). αC:αC helix, whose position tilted toward the kinase C-lobe, is critical for the correct positioning of ATP in the substrate-binding cleft. (F) Mutations that disrupt the KD-AID interaction make AMPK constitutively active. Wild-type (WT) and mutant KD-AID (α1 11-353) were incubated with His6GST-SAMS peptide (SAMS) substrate and [γ-32P]-ATP. Terminated reactions were separated by SDS-PAGE and subjected to autoradiography. See Supplementary information, Figure S7 for repeat experiments and SD. (G) Mutations that disrupt the KD-AID interaction do not affect AMP-dependent stabilization of activation loop phosphorylation. Phosphorylated WT and mutant AMPK were incubated with human PP2Cα in the absence or presence of 0.2 mM AMP for the indicated amount of time, separated by SDS-PAGE, and subjected to immunoblotting with an antibody specific for T174-phosphorylated AMPK. See Supplementary information, Figure S7 for repeat experiments and SD.