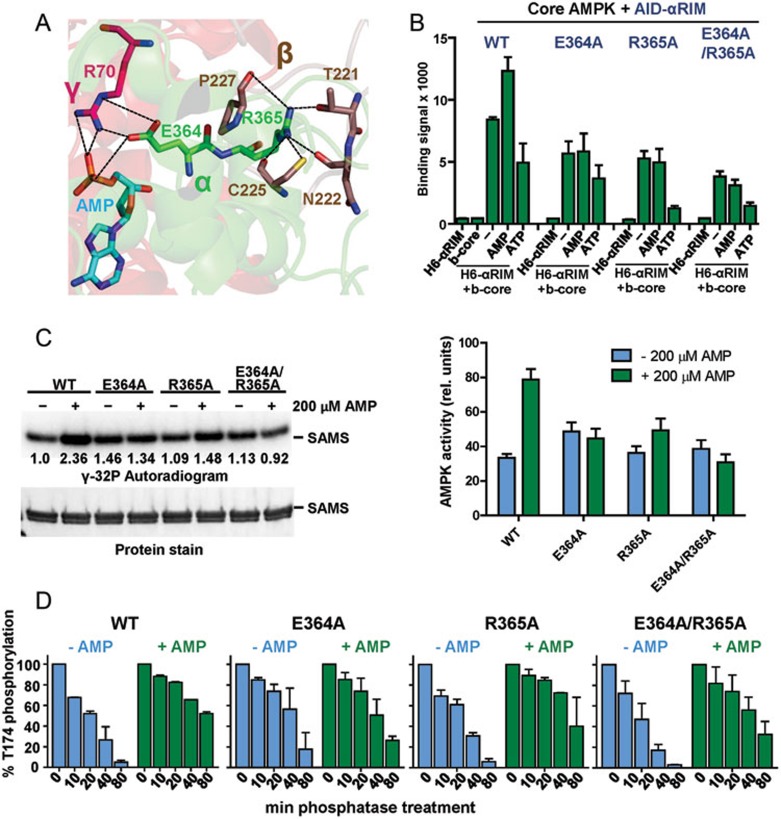
Figure 5.
Adenine nucleotide-mediated conformational changes in AMPK depend on inducible α-RIM-CBS3 and AID-KD interactions. (A) Interaction network between AMP, CBS-3 R70, αRIM-2 E364 and R365, and 4 residues from an induced loop of the β-subunit. AMP and key amino acids are shown in stick presentation with bonds indicated as dashed lines. (B) AMP increases and ATP decreases the interaction between AID-αRIM and core AMPK(α1[395-550]-β2[187-272]-γ1). AlphaScreen interaction between biotinylated core AMPK and wild-type (WT) and mutant His6GST-AID-αRIM in the presence and absence of adenine nucleotides. (C) Mutations that disrupt the α-RIM-CBS3 interaction abolish direct AMP regulation of AMPK catalytic activity. Radioactive kinase assay showing 32P incorporation into SAMS substrate (left, representative autoradiogram; right, quantification of 32P incorporation based on three independent experiments (error bars, SD). (D) Mutations that disrupt the α-RIM-CBS3 interaction reduce AMP-mediated protection against activation loop dephosphorylation. Bars represent relative AMPK T174 phosphorylation as determined by immunoblot, following the indicated time of incubation with PP2Cα (error bars, SD; n = 3).