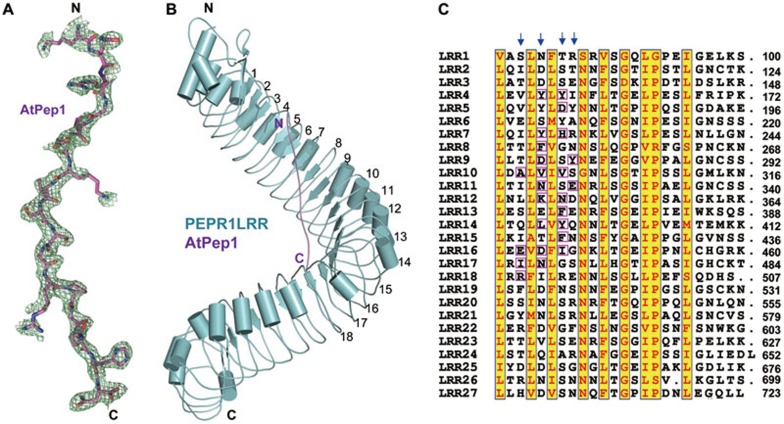
Figure 2.
AtPep1 binds to the inner surface of PEPR1LRR superhelical structure. (A) Electron density around AtPep1 in the structure of the PEPR1LRR-AtPep1 complex. Omit electron density (2Fo-Fc) around AtPep1 (residues 7-23) contoured at 1.20. “N” and “C” represent the N- and C-terminus, respectively. Color codes are indicated. (B) Overall structure of the PEPR1LRR-AtPep1 complex shown in cartoon. The positions of some LRRs are indicated. (C) Sequence alignment of LRRs of PEPR1LRR. Numbers indicate the positions of the last amino acid of each LRR. The conserved residues are shown with yellow background. Amino acids involved in interaction with AtPep1 are highlighted with magenta frames. Arrows indicate the 3rd, 5th, 7th and 8th positions of each LRR.