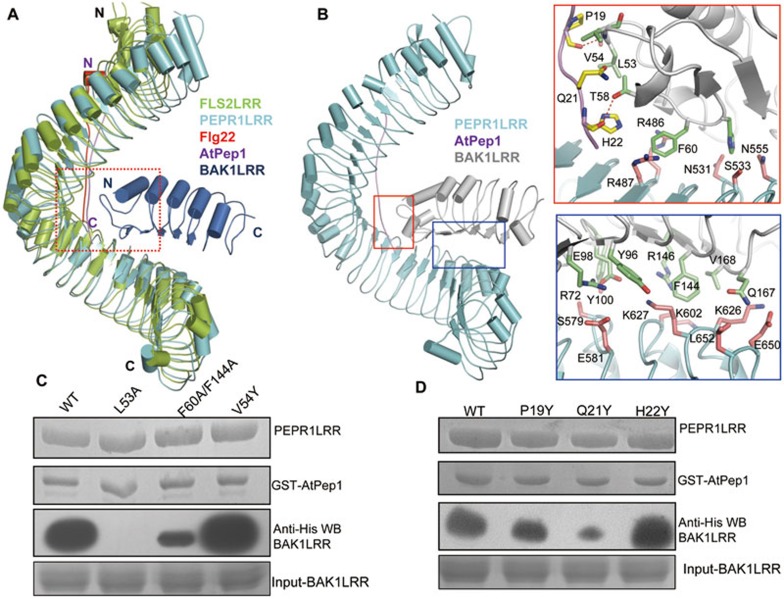
Figure 4.
Modeled structure of the PEPR1LRR-AtPep1-BAK1LRR complex. (A) Structural comparison of PEPR1LRR-AtPep1 with FLS2LRR-flg22-BAK1LRR (PDB code: 4MN8). The C-terminal 20 LRRs of PEPR1LRR were used to aligned with those of FLS2LRR. Color codes are indicated. (B) AtPep1 mediates PEPR1LRR-BAK1LRR heterodimerization. Left panel: overall modeled structure of the PEPR1LRR-AtPep1-BAK1LRR complex shown in cartoon. Right top: interaction of the C-terminal portion of AtPep1 with BAK1LRR. The side chains of PEPR1LRR, AtPep1 and BAK1LRR are shown in pink, yellow and green, respectively. Right bottom: PEPR1LRR and BAK1LRR make direct contacts. (C) Mutations in BAK1 affect AtPep1-induced PEPR1LRR-BAK1LRR interaction. The assay was performed as described in Figure 1A. (D) The mutation AtPep1Q21Y compromises AtPep1-induced PEPR1LRR-BAK1LRR interaction but has little effect on recognition of the peptide by PEPR1LRR. The assay was performed as described in Figure 1A.