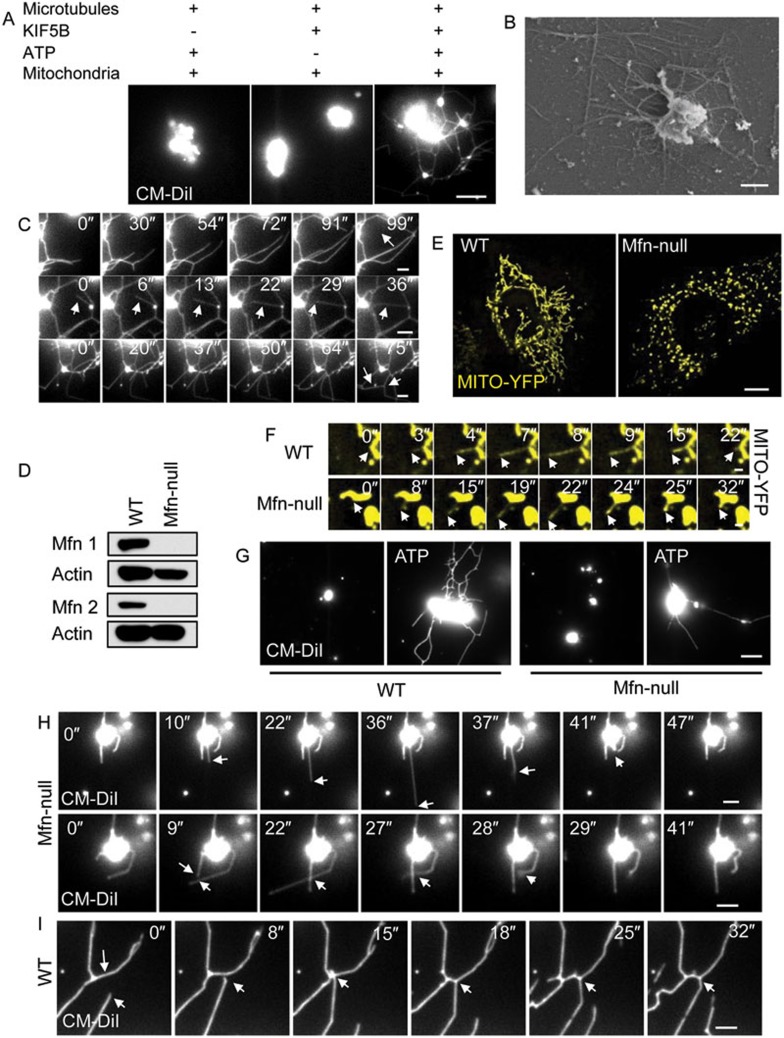
Figure 6.
KIF5B and mitofusins drive mitochondrial network reformation in vitro. (A) Purified mitochondria were labeled with CM-DiI. Highly concentrated mitochondria were incubated with KIF5B, transferred into flow chamber channels coated with polymerized microtubules, and visualized in the presence of ATP. Images were collected with a TIRF microscope. Scale bar, 5 μm. (B) Samples from A were analyzed by FEISEM. Scale bar, 1 μm. (C) Time-lapse sequence of A. Scale bar, 2 μm. White arrows indicate newly formed lattices (top and bottom rows) or a tubule that divides a lattice (middle row). (D) Mfn1 and Mfn2 levels in Mfn-null MEF cells and wild-type (WT) MEF cells as shown by western blotting. (E) WT MEF cells and Mfn-null MEF cells were transfected with Mito-YFP and observed by spinning-disk microscopy. Scale bar, 10 μm. (F) Time-lapse sequence of mitochondrial dynamic tubulation in WT (top row) and Mfn-null MEF cells (bottom row). Scale bar, 1 μm. (G) Mitochondria from WT and Mfn-null MEF cells were tested in the in vitro reconstitution system for their ability to form mitochondrial networks. Scale bar, 5 μm. (H) Time-lapse sequence of tubulation of mitochondria from Mfn-null MEF cells. Scale bar, 2 μm. White arrows indicate pull-back of an unstable dynamic tubule (top row) or two crossed tubules that cannot fuse with each other (bottom row). (I) Time-lapse sequence of tubulation of mitochondria from WT MEF cells. Scale bar, 2 μm. White arrows indicate a fusion event.