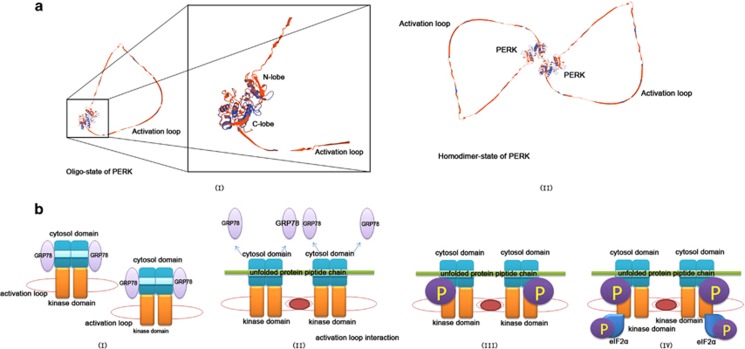
Figure 1.
Molecular structure of PERK kinase domain and 'line-up' activation mechanism. (a) The presumed three-dimensional (3D) molecular model of PERK kinase domain. (I) The oligostate of PERK kinase domain. This domain could be divided into N-lobe and C-lobe, which are connected by a short hinge loop. There is a distinctive long activation loop in this structure. (II) Two individual PERK molecules dimerize to establish the homodimer state of PERK kinase domain, which is the most common form of PERK. (b) (I) At resting state without ER stress, GRP78 binds to the cytosol domain of dimerized PERK. (II) Under external harmful stimuli, the unfolded protein accumulate in the ER lumen to initiate ER stress by disassociating GRP78 from PERK. Then, the peptide chain of an unfolded protein bind to MHC-like grooves of the cytoplasmic domain to 'line up' the stacked PERK dimers. (III) The activation loop of neighboring PERK kinase domain interact with each other to trigger autophosphorylation of PERK. (IV) eIF2α are recruited and phosphorylated by activated PERK