Figure 7. Structural compatibility of diverse mammalian PrPs within amyloid fibrils based on in-register cross β-sheet of PrP 90-231.
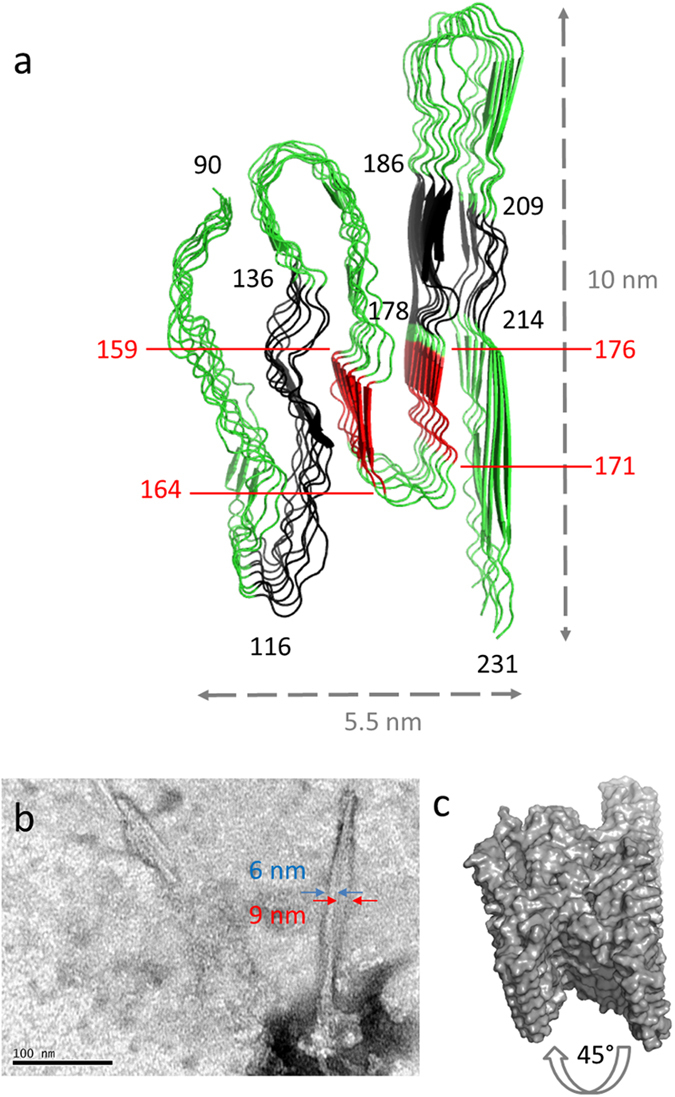
a) Conformational compatibility within the fold of PrP within an amyloid fibril. Black colors denote aggregation prone sequences from AGGRESCAN and red colors denote predicted amyloidogenic sequences by WALTZ (as shown in Fig. 6). The figure was made using PyMol and the pdb coordinates PIRIBS-A-EM-ND.pdb including eight protomer chains from the model proposed by Groveman et al. 2014 based on HaPrP90-231. b) Negative stain TEM of an individual fibril of HuPrP23-231 from a self-seeded reaction shows a morphotype compatible with the proposed single protein molecule cross section of the model in a similar to that reported by Groveman et al. 2014 for brain derived fibrils from MoPrP. c) Surface rendering of a tilted fibril model (same as in a) to demonstrate the compatibility of the structural model with the experimental data in b.
