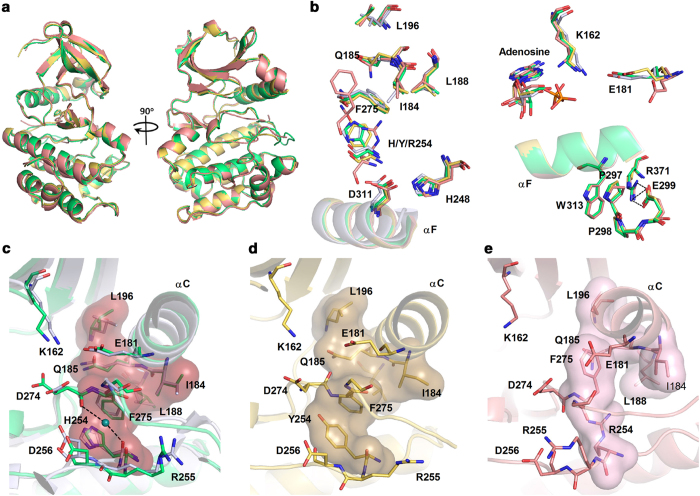
Figure 2.
The conserved pattern of the catalytic core and the R-spine are disrupted by the HxD-histidine mutation. (a) Overall structure comparison of WT Aurora A and the HxD-histidine mutants. (b) Structural alignment of key elements that define the active conformation of EPK. For comparison, fully activated conformation of Aurora A (with phosphorylated activation segment, PDB code: 1MQ4) is used to align with our WT Aurora A and the mutants. Both our WT Aurora A and the H254Y mutant superpose well to the fully activated Aurora A, while the R-spine is disrupted in H254R mutant. (c-e) Detail views of the critical residues in the catalytic cores of WT Aurora A, the H254R and the H254Y mutants. The carbons of the WT, H254R and H254Y Aurora A proteins are colored green, pink and yellow, respectively. The fully activated conformation of Aurora A is colored gray. R-spine and the hydrophobic hook are shown as surface model. The conserved water molecule in the catalytic core of the activated Aurora A is shown as blue sphere. Polar interactions are shown as black dash lines.