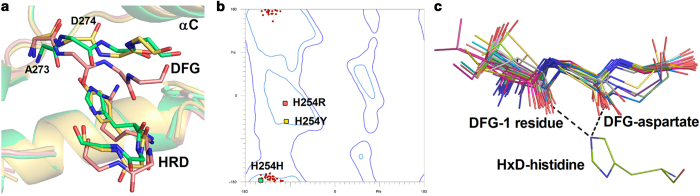
Figure 3.
The HxD-histidine is involved in the preservation of the active conformation of xDFG motif. (a) Conformational changes of the HxD- and xDFG-backbones in the H254R and H254Y mutants. Only the side chains of H/R/Y254 are shown as stick models for clarity. The carbons of the WT, H254R and H254Y Aurora A proteins are colored green, pink and yellow, respectively. (b) Ramachandran plot shows the conserved conformation of the xDFG-backbone in active EPKs. Dihedral angles of the DFG-1 residues in the active conformations listed in Table S1 are indicated as red dots. Dihedral angles of A273 in WT as well as the H254R and H254Y mutants are indicated as green, pink and yellow squares, respectively. (c) Superposition of the xDFG-backbones of the active structures listed in Table S1. For clarity, only the HxD-histidine of Aurora A is shown in line model. Hydrogen bonds between the HxD-histidine and xDFG-backbone are shown as black dash lines.