Figure 5.
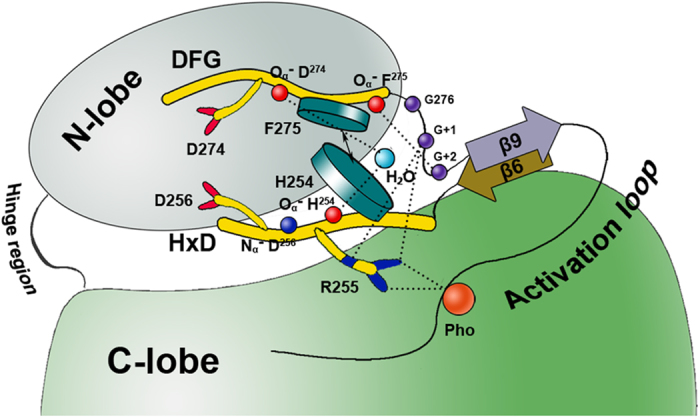
A schematic diagram showing the conserved inflexible organization of the catalytic core (Aurora A is used as the example). This pattern is conserved through the active structure listed in Table S1. The hydrophobic interaction between the HxD-histidine and the xDFG-phenylalanine packages up the HxD and the xDFG motifs. The hydrogen bonds among the HxD-histidine, the xDFG-backbone, and the conserved water molecule in the catalytic core are involved in the conformational maintenance of the catalytic core. The hydrogen bonds among the HxD-arginine, the xDFG-aspartate, G + 1 and G + 2 residues have been considered to be required for the catalytic activity. Carbons in the HxD and the xDFG motifs are colored yellow. The key atoms in the HxD and xDFG motifs are shown as sphere models. The G276, W277 and S278 residues are shown as purple spheres. The conserved water molecule and the phospho-site are shown as blue and orange spheres, respectively.
