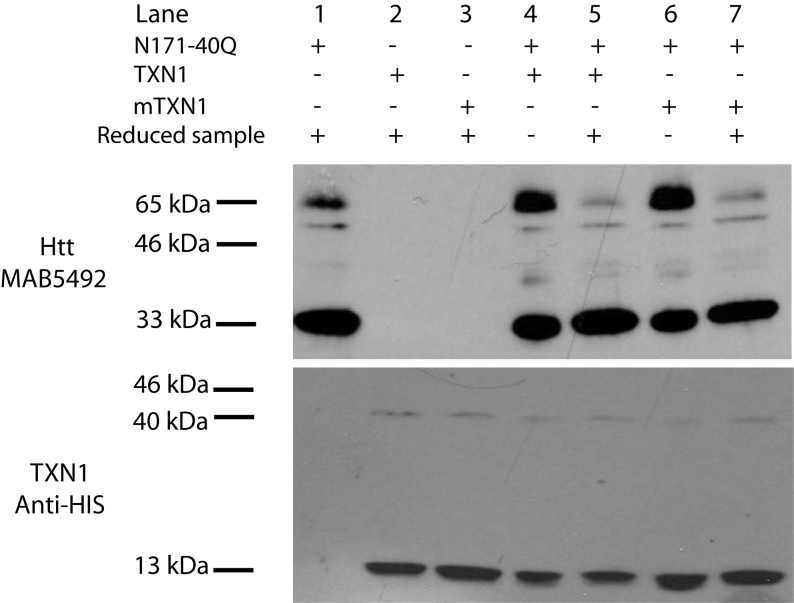
TRX1 does not interact directly with N171-40Q mutant huntingtin in a cell-free assay.
TRX1, mutant TRX1 (C35S) and N171-40Q were expressed and purified from bacteria. N171-40Q was incubated with TRX1 or mTRX1 at 250 C for 1 hour then free thiols blocked with 50 mM N-ethylmaleimide. Samples were then analyzed by SDS-PAGE (see methods). Lanes: 1=N171-40Q htt alone; 2=TRX1 alone; 3=mTRX1 alone; 4-5=N171-40Q and TRX1; and 6-7= N171-40Q and mTRX1. Mutant TRX1 (C35S) is predicted to trap substrate proteins as heterodimers [33]. There is no evidence of a heterodimer band (estimated mass = 46 kDa) in the non-reduced N171-40Q and mTRX1 group (lane 6). The band migrating at ~65 kDa (most prominent in lanes 4 and 6) is dimeric N171-40Q as previously reported6.