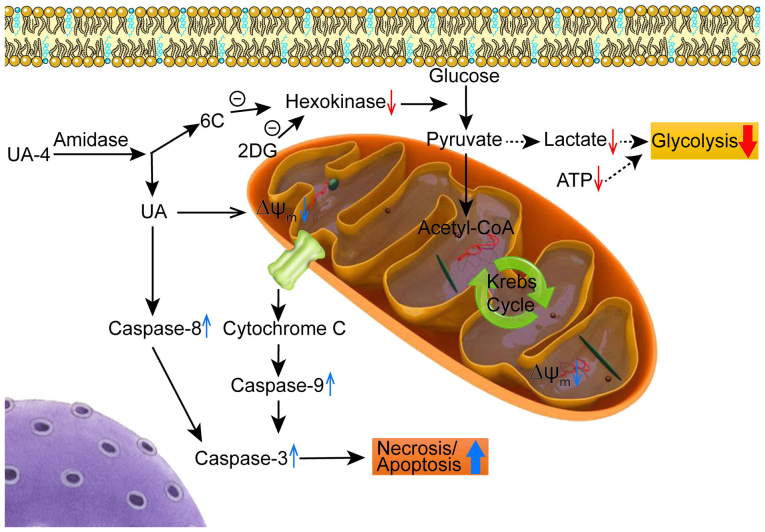
Figure 7. Proposed mechanisms by which the leading UA derivative UA-4 and 2-DG produce synergistic inhibition on cancer cell proliferation.
After entering the cells, UA-4 is hydrolyzed by amidase to be broken down to the carbon chain 6C and UA metabolite. The former competes with glucose for binding to hexokinase to inhibit cancer glycolysis pathway, while the latter produces depolarization in ΔΨm, cell arrest, ATP consumption, necrosis/apoptosis via caspase-independent pathway. 2-DG also inhibits glycolysis of cancer cells by targeting hexokinase. The dual targeting of cell necrosis/apoptosis and glycolysis pathways by using the safe and effective molecules represents a novel anticancer agent.