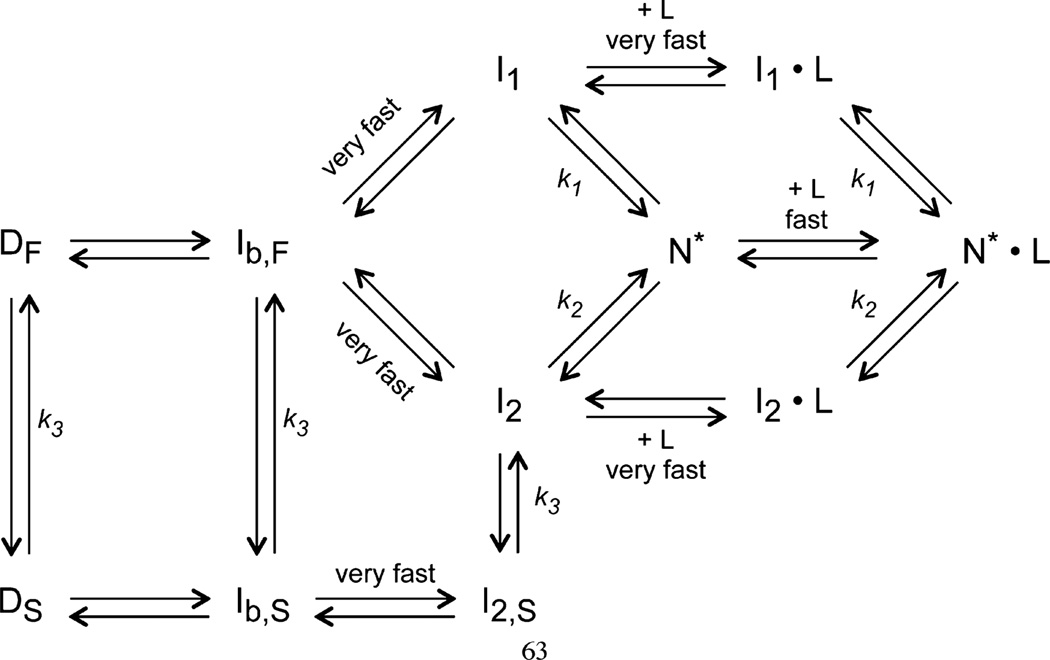
Figure 10.
Folding–ligand binding kinetics of the leave-one-out GFP. Proposed model of the folding–ligand binding mechanism of the leave-one-out GFP, modified from ref 58 by the addition of the three ligand binding steps. Two unfolded species (DF and DS) are equilibrated through a slow prolyl isomerization and fold to the intermediate states (I1 and I2) after the initiation of refolding. In the presence of ligands (L), both intermediate species form complexes (I1•L and I2•L) rapidly and further fold to the native state (N*•L). Notice that both intermediates can also fold to a different native state (N*) without the presence of ligands, and N* can further bind to ligands and convert to the N*•L state. Both N* and N*•L are the fluorescent species, by definition.