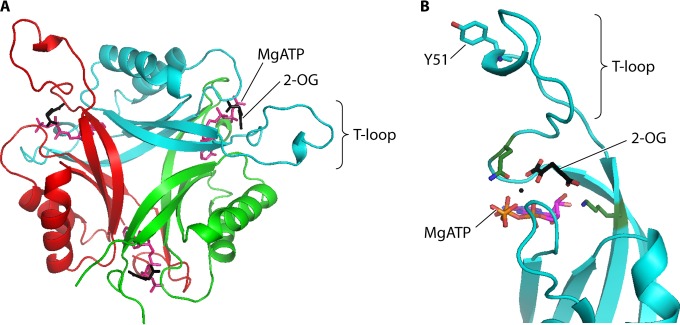
FIG 2.
PII protein structure. (A) PII trimer. The binding sites for MgATP (magenta) and 2-OG (black) at the lateral clefts between each subunit are indicated. (B) Closer view of the 2-OG binding site. 2-OG interacts with the side chains of conserved lysine (K58) and glutamine (Q39) residues (green sticks) and also with MgATP. 2-OG binding affects the PII T-loop structure, thereby altering the affinity between PII and its binding protein targets. In proteobacteria, the structure of the T loop is also influenced by reversible uridylylation at the conserved tyrosine residue indicated (Y51). The figure was prepared using Pymol and PDB entry 3MHY.