ABSTRACT.
The goal of radiotherapy is to achieve uniform target coverage while sparing normal tissue. In proton therapy, the same sources of geometric uncertainty are present as in conventional radiotherapy. However, an important and fundamental difference in proton therapy is that protons have a finite range, highly dependent on the electron density of the material they are traversing, resulting in a steep dose gradient at the distal edge of the Bragg peak. Therefore, an accurate knowledge of the sources and magnitudes of the uncertainties affecting the proton range is essential for producing plans which are robust to these uncertainties. This review describes the current knowledge of the geometric uncertainties and discusses their impact on proton dose plans. The need for patient-specific validation is essential and in cases of complex intensity-modulated proton therapy plans the use of a planning target volume (PTV) may fail to ensure coverage of the target. In cases where a PTV cannot be used, other methods of quantifying plan quality have been investigated. A promising option is to incorporate uncertainties directly into the optimisation algorithm. A further development is the inclusion of robustness into a multicriteria optimisation framework, allowing a multi-objective Pareto optimisation function to balance robustness and conformity. The question remains as to whether adaptive therapy can become an integral part of a proton therapy, to allow re-optimisation during the course of a patient's treatment. The challenge of ensuring that plans are robust to range uncertainties in proton therapy remains, although these methods can provide practical solutions.
The ability to create and deliver the ideal treatment plan, where the target volume receives 100% of the prescribed dose and normal tissue receives 0%, is the holy grail of radiation therapy [1]. It is, however, impossible to achieve this perfect balance. Instead, multiple trade-offs are required to achieve a clinically acceptable plan, so the problem becomes one of optimisation. There are many factors that can affect how “optimised” a patient's treatment can be. This review focuses on the challenges of proton therapy plan optimisation, particularly in regard to range uncertainties, and how to incorporate them into the plan evaluation and verification process.
The nature of proton therapy makes the aim of cure without complications potentially more achievable, owing to the highly localised deposition of dose in the characteristic Bragg peak [2]. This relates predominantly to the ability to deliver high doses of radiation close to normal tissue structures, which would be dose limiting in conventional X-ray treatments, and to the finite range of protons, which results in a reduced integral dose to surrounding normal tissues.
From a clinical perspective, the exact role of proton therapy has yet to be defined. However, for childhood cancers, proton therapy delivers a lower dose to tissues around the tumour than X-rays, resulting in less growth disturbance and lower risk of secondary malignancies. There is also the suggestion that the use of proton therapy can reduce impairment of neuropsychological and intelligence quotient development [3]. In adults, proton therapy seems particularly effective in the treatment of radio-resistant tumours close to critical structures such as the brain stem and spinal cord. For example, outstanding results have been published for the use of proton therapy in the treatment of chordoma and chondrosarcoma [4]. The current evidence for the use of proton therapy at different sites has been extensively reviewed [5-8]. However, it will also be important to consider expanding potential indications where logic and dosimetry indicate that proton therapy can confer an advantage [7]. Although clinical results comparing proton therapy with the most modern X-ray therapy are currently lacking, overall clinical outcomes are promising for both delivery options and support the rationale for proton therapy [8]. The reader is referred to the literature [9-11] for more clinical data. Nevertheless, substantial opportunity for further clinical research development and evaluation remains.
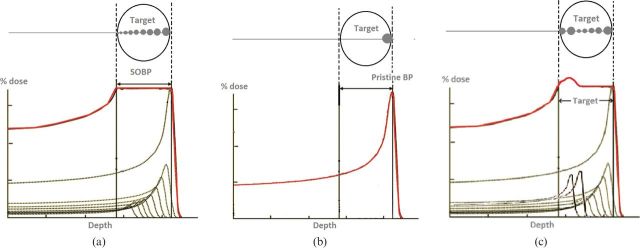
The choice of treatment delivery can have a large impact on the ability both to produce conformal dose distributions and to produce a plan which is robust to uncertainties. There are three main treatment delivery techniques used clinically: passive scattering [12], uniform scanning and active scanning [13,14] (Table 1). These techniques are used to broaden the narrow proton beam created by the accelerator into one that can achieve a uniform dose coverage of the target at all depths. This is achieved for passive scattering and uniform scanning through the delivery of so-called spread-out Bragg peaks (Figure 1).
Table 1. Proton beam delivery techniques, production methods and planning techniques (the further down the table, the more conformal the technique).
| Methods of producing a clinical proton beam to treat entire target volume | Descriptions |
| Passive scattering | Works on the principle that high atomic number materials, such as lead, scatter the beam with minimum energy loss and low atomic number materials, such as plastic, decrease proton energy with minimum scatter. Combining these materials to produce patient-specific collimators and compensators results in a conformal treatment beam with a spread-out Bragg peak |
| Uniform scanning | This is similar to passive scattering with the difference that the beam is spread in the lateral direction through magnetically deflecting the beam with constant fluence instead of using a scattering foil. Different spot weights are produced using a compensator, as in passive scattering |
| Active scanning | This uses magnetic fields to deflect the path of each proton beam towards the planned position in the target volume. Individual Bragg peaks are distributed within the target volume and the cumulative effect produces an effective SOBP without the need for compensators. This is achieved by either continuous magnetic scanning or spot scanning. The latter is analogous to the step-and-shoot mode in IMRT, i.e. a non-continuous delivery of dose, where the exact position is determined before the dose is delivered |
| Methods of achieving adequate dose distributions | Descriptions |
| SFUD | Single individually optimised proton fields that each deliver a homogeneous dose to a volume. If necessary, these can be combined by simple addition |
| Field patching | The sharp distal edge dose gradient can be matched up to the lateral edges of another “patch” field to produce a continuous dose distribution. Where possible, equivalent opposite fields are also used to reduce the potential for dose variation at the abutting edges. Multiple fields in patch work can be used to achieve multiple dose gradients inside a treatment volume. Field patching is a 3D extension of matching lateral field edges. Therefore, if multiple fields are used, each one can deliver a homogeneous dose to part of the volume |
| IMPT | IMPT is analogous to IMRT, and is a mode of treatment delivery achievable only with active scanning beams. IMPT uses narrow proton beams which are magnetically moved over the volume in the transverse plane while the energy and intensity are altered to control dose to a point and sculpt the dose at depth. Unlike SFUD treatments, IMPT can deliver a number of non-uniform fields to produce the desired dose distribution |
| “Flavours” of IMPT | Descriptions |
| 3D IMPT | This is most similar to IMRT. Bragg peaks are placed throughout the entire volume and their weights optimally adjusted |
| DET | DET is a method by which pristine Bragg peaks of optimal weights are distributed only along the distal edge of the target and not throughout the target volume |
3D, three-dimensional; DET, distal-edge tracking; IMPT, intensity-modulated particle therapy; IMRT, intensity-modulated radiotherapy treatment; SFUD, single-field uniform dose; SOBP, spread-out Bragg peak.
Figure 1.
Schematic of Bragg peak delivery along a single profile through a target. (a) A flat spread-out Bragg peak (SOBP) is achieved by placing spots with increasing weights throughout the target to produce a uniform field, as used in passive scattering and single-field uniform dose. (b) Only the most distal single pristine Bragg peak (BP) is used for distal-edge tracking. (c) Optimally weighted spots are positioned throughout the volume to achieve fields with non-uniform doses for three-dimensional intensity-modulated particle therapy.
Orthogonal to the beam direction, the beam is spread using carefully designed scatterers (for passive scattering) or by continually deflecting the proton beam in a regular pattern orthogonal to the beam direction with constant intensity (uniform scanning). For both approaches, three-dimensional (3D) conformation of the final dose to the target is achieved through the additional use of patient- and field-specific collimators (which conform the dose in directions orthogonal to the beam) and compensators (which conform the dose in the beam direction) inserted in the beam nozzle [14]. Active scanning, on the other hand, also uses magnets to scan the proton beam across the target volume, but, in contrast to uniform scanning, allows the fluence (dose) applied at each Bragg position to be continuously varied. Active scanning can offer an advantage to the patient by allowing for greater flexibility in the delivered dose and a reduction in integral dose to healthy tissues. It also allows for the delivery of intensity-modulated particle therapy (IMPT) [15], which is analogous with intensity-modulated radiotherapy treatment (IMRT) in conventional radiotherapy. Although there is, in principle, a continuum of solutions to the IMPT problem, at its extremes, IMPT can be divided into two “flavours”: distal-edge tracking (DET) [16], where Bragg peaks are placed only at the edge of the target volume, and 3D IMPT [15], where Bragg peaks are optimally distributed and weighted throughout the target volume (Figure 1). IMPT allows for delivery of single inhomogeneous but optimised fields to produce a final inhomogeneous dose distribution in the target volume. This permits the planner to be more flexible in the placement of residual dose to healthy tissues. However, although IMPT offers greater optimisation of dose delivery at the planning stage, it has the potential to be sensitive to range uncertainties [17,18]. Table 1 summarises all these modes of producing and manipulating proton dose distributions.
Uncertainties in proton planning
Sources of uncertainty present in conventional radiotherapy also apply to proton therapy. Most geometrical uncertainties can be managed in the same way, including variation in delineation, set-up uncertainties, imaging inaccuracies and patient motion. However, there exists an important and fundamental difference in proton therapy—the proton range. Protons have a finite range, highly dependent on the electron density of the material they are traversing, resulting in a steep dose gradient at the distal edge of the Bragg peak. Positioning of these dose gradients is critical to successful planning and treatment. Therefore, an uncertainty of even a few millimetres can lead to underdosage in the target volume or overdosage of an organ at risk (OAR).
Several authors have addressed the problem of range uncertainties in proton therapy, and the purpose of this section is to analyse their conclusions. Understanding the causes and magnitude of range uncertainties and incorporating them into the planning process is essential for optimised proton planning.
Sources of range uncertainty
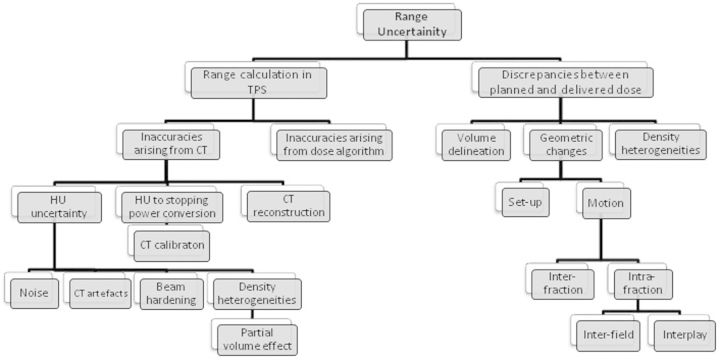
The main factors leading to range uncertainty are shown in Figure 2. Because the main advantage of using protons in cancer treatment is their finite range, this advantage can be fully exploited only if the proton range in the patient can be precisely predicted [19]. It has been suggested that range uncertainties can be between 1 and 15 mm for lung tumours [20], but larger changes are possible owing to anatomical changes in the patient (e.g. weight loss or gain and differential filling of anatomical cavities). Uncertainties are normally compensated for in X-ray radiotherapy by introducing safety margins around the treatment volume and around OARs to produce a planning target volume (PTV) and planning OAR volume (PRV), as recommended by the International Committee on Radiation Units and Measurements (ICRU) Reports 50 and 62 [21,22]. A similar method has been recommended by the ICRU for protons [23]. The larger the safety margin, the less conformal the resulting dose distribution. Therefore, to achieve an optimum proton treatment plan, the range prediction needs to be as accurate as possible.
Figure 2.
Schematic of sources of range uncertainties in proton radiotherapy. HU, Hounsfield unit; TPS, treatment planning system.
The variables that give rise to uncertainties in the range prediction (Figure 2) can be divided into two main groups: those causing uncertainties in the range calculation in the treatment planning system (TPS), and those leading to discrepancies between planning dose and delivered dose.
Range calculation in the treatment planning system
Inaccuracies arising from the planning CT
With respect to range calculation uncertainties, these can arise from inaccurate data exported to the TPS. CT is used to acquire patient image data and the Hounsfield units (HUs) are then converted into proton-stopping powers so dose calculations can be made. Errors arise in proton range calculation from CT-based plans owing to inaccuracy in the HU to proton stopping power conversion and inaccuracies in the HU values themselves [24]. Inaccuracies in the HU values are caused by noise, CT artefacts and beam hardening.
Schaffner and Pedroni [25] and España and Paganetti [20] investigated how the conversion of HUs to stopping power affects the range calculation in comparison with the real treatment range. España and Paganetti [20] tested different conversion methods, including the traditional stoichiometric calibration method. Positron emission therapy (PET) imaging was used to determine the range of the proton beam in a phantom and compare it with the calculated range. It was noted that the back-to-back photons imaged with PET are generated from electron–positron annihilations caused by inelastic nuclear interactions between protons and target nuclei and not from atomic interactions, which primarily leads to dose deposition. España and Paganetti [20] and Schaffner and Pedroni [25] both concluded the same result: that the uncertainty caused by conversion was <±1%. There is research being undertaken into the development of proton CT. This would remove the uncertainty in the conversion from HUs to proton-stopping powers and enable image guidance with the patient set-up for treatment [26].
Noise is a stochastic error that either adds or subtracts from the HU value; this type of error is important only if the proton beam is sensitive enough to be affected by these changes in HU value. It was concluded that errors caused by noise have a similar contribution to conversion uncertainties: <±1% [25]. Beam hardening had a greater effect on the assigned HU value. This was dependent on the position and density of the tissue and added errors of the order of ±1.8% and ±1.1% for bone and soft tissue, respectively [25].
It is essential for CT-scanner-specific calibrations to be carried out [23,25]. This is because, even though stopping power is independent of proton energy and the position of the target, HUs are dependent on the X-ray spectrum and target position. Each scanner will produce a different X-ray spectrum generated with a different tube potential and current, therefore requiring individual calibration. Moyers et al [27] carried out the measurement of the relative linear stopping powers of 21 different tissue substitutes. These were then scanned using both kilovoltage and megavoltage CT and the relationship between stopping power and HU value was determined.
Lomax [28] has described how the combined effects of the proton's sharp distal fall-off, finite range and multiple Coulomb scattering can have an impact on the sensitivity of plans to density heterogeneities in the patient. The Paul Scherrer Institute in Switzerland has biologically calibrated its CT scanner to have an accuracy of 1% for soft tissue and 2% for bony tissue, but, owing to inherent errors such as beam hardening, reconstruction artefacts and reconstruction algorithms [28], it has been stated that an error in HU value of 3% is more realistic. To investigate the effect of this error, each plan was recalculated with the HU value increased and decreased by 3% to simulate an undershoot and overshoot scenario. The results were obtained for a simple prostate case and a skull base case. The distal-edge tracking (DET) approach was found to incur a systematic over- and underdosage of the clinical treatment volume (CTV) of ∼5% when a ±3% HU error was introduced, whereas the 3D IMPT dose–volume histogram (DVH) for the nominal, under- and overshoot plans showed little difference.
Inaccuracies arising from the dose calculation algorithm
Lomax [19] investigated the limitations in analytical dose calculations and the effects of uncertainties in density calculation from CT data in DET and 3D IMPT dose distributions. To investigate errors arising from using an analytical dose calculation algorithm the dose distributions for Version 1 and Version 2 DET and 3D IMPT skull base plans were compared with the same plans calculated using Monte Carlo models (Version 1 plans use less stringent constraints on OARs than Version 2 plans). In contrast to using an analytical mathematical algorithm to calculate dose distributions, Monte Carlo models use a probability distribution to model interactions and the production of secondary particles in a medium for a given energy. For the skull base case the two calculation methods were in close agreement for an acceptance level of ±10%, but decreased for lower acceptance levels. At all acceptance levels, 3D IMPT plans showed better agreement in the PTV than DET plans, with 87% of points in the 3D IMPT plan agreeing to within ±3% and only 80% of points agreeing to within ±3% in the DET plan for Version 1 plans. However, for the Version 2 plans, agreement falls to 77% and 70% for 3D IMPT and DET, respectively. In the OARs it was found that the Monte Carlo calculation predicted smaller doses in the optic nerve and brain stem than the analytical calculation, especially in the Version 2 plans. The trend showed that for both cases 3D IMPT showed smaller differences than the DET plans. It was concluded that DET IMPT was relativity sensitive to calculation errors; in comparison, 3D IMPT was more robust with respect to both types of error.
Discrepancies between planned dose and delivered dose
Despite algorithms being able to calculate range in the presence of density heterogeneities, range uncertainties can be introduced by geometric changes in the position of density heterogeneities relative to the proton beam by set-up errors and patient motion [16].
Motion
In many treatment sites, organ motion has to be considered and incorporated into the planning and delivery workflow. Organ motion includes interfraction motion (i.e. between each fraction) and intrafraction motion (i.e. during the treatment delivery). Patient motion influences the position of the interfractionally moving organ and intervention in the form of immobilisation and image guidance for precise bony or soft-tissue anatomy set-up at the beginning of each fraction is required to optimise treatment delivery and minimise CTV to PTV margins. These are well-established techniques in radiotherapy [29], so will not be discussed further.
Intrafraction motion includes both interfield (i.e. between each field) and intrafield (i.e. during the field) motion. Intrafraction motion changes during the delivery over the time period of seconds and minutes and includes anatomical changes such as bowel movements, respiration and heartbeats. Studies have been carried out to determine the magnitude of inter- and intrafraction motion at different tumour sites [30-32].
With proton therapy, geometric changes caused by motion can also result in density changes, and therefore a change in radiological path length, along the beam path. In X-ray therapy, the dose distribution changes by only a few per cent, owing to density changes. However, their influence in proton therapy can result in severe underdosage of the CTV and overdosage of OARs and normal tissues distal to the target [33]. This effect is the same for both scattering and scanning delivery techniques. In addition, for active scanning, the major effect of intrafield motion is “interplay”, which relates to motion, usually respiratory motion, with a frequency similar to that of the scanned beam, and which can lead to over- and underdosage in the target volume [34-37].
Lomax [19] investigated the effects of interfraction, intrafraction and interfield motion for both 3D and DET IMPT treatment plans. It was reported that, for a 5-mm shift in the dose distribution, an underdosage of up to 20% can occur in the CTV when plan optimisation for maximum OAR sparing is used. Treatment deliveries involving high dose gradients that rely on matching contributing fields are very sensitive to any changes in position between deliveries of each field. An important conclusion from this paper is that for certain IMPT plans a simple PTV margin cannot be applied to compensate for interfraction motion. Further investigation into the management of uncertainties and more assessment for IMPT treatments at the treatment planning level are needed. Lomax [19] also described the greater sensitivity protons have to density heterogeneities owing to their physical characteristics and that these could accentuate motion errors. Without a PTV, there is no method for recording dose to a moving CTV or for evaluating plans through the use of DVHs. Without a PTV, there is no method for recording close to a moving CTV or for evaluating plans through the use of DVH's and for these IMPT plans, no other compensation method to ensure that the CTV is covered. After comparing the effects of geometrical and density heterogeneities for both DET and 3D IMPT it was found that DET plans are very sensitive to motion errors and considerable changes in the dose distribution were found. The reasoning behind this is that internal dose gradients in the individual fields in DET can cause large variations in dose within the target volume when mismatched. This was observed for interfield motions, but would also be an issue when intrafield motions were present.
Simulations have also been carried out by Lambert et al [33], albeit only in homogeneous geometries, to investigate how interplay affects the dose distribution in the CTV. Lambert et al took the ICRU 50 [21] recommendations for PTV dose homogeneity of 95–107% as threshold. Their results showed that in extreme cases up to 100% of the target volume received doses below that recommended by ICRU 50 and with a minimum dose as low as 34% of the prescribed dose. These results were backed up by simulations carried out by Grözinger et al [38] and experimental work by Bert et al [39]. Bert et al carried out the first patient simulation that confirmed underdosage using four-dimensional computed tomograph (4DCT) lung data. Despite using margins that consider the effect of the changing radiobiological path length, adequate CTV coverage could not be achieved.
There also exists a related range uncertainty owing to the relative biological effectiveness (RBE) of proton beams, which is beyond the scope of this review. For more information on RBE the reader is referred to the literature [40-43].
Managing uncertainties including positional discrepancies and motion
The management of uncertainties is critical to successful radiotherapy. Current methods of reducing geometric uncertainties include immobilisation, in-room imaging, image guidance, planning from 4D CT and gated radiotherapy for respiratory motion. These are all methods that are routine in X-ray therapy and are now being applied to proton therapy [44].
Motion mitigation
Two main methods of motion mitigation have been developed for active scanning to mitigate the effects of interplay: rescanning (repainting) and beam tracking [44].
Rescanning
Typically in proton therapy, multiple dose “painting” is required to deliver the prescribed dose distribution to each layer of the target volume. In IMPT, the dose is delivered from multiple directions by a number of fields and with a range of different proton energies to produce a uniform dose distribution throughout the target volume. To increase dose conformity, steep dose gradients are used at the target border and field edges. This increases the complexity of the fluence maps per field and therefore makes the plan less robust to uncertainties. Intrafraction motion can lead to an under- and overdose pattern that is dependent on the motion parameters (initial phase, period and amplitude) and the speed of the scanning process or direction of scanning. By rescanning the PTV several times per treatment fraction an averaging effect of the over- and underdose pattern can be achieved. As long as the intrafraction motion changes between each rescan, so that there is no synchronisation between delivery and organ motion, a homogeneous dose distribution to the CTV can be achieved with a “blurred” dose distribution in the region of the margins [44]. There are two main types of rescanning:
rescanning by energy slice, also known as slice-by-slice, level painting or non-volumetric rescanning
rescanning of volume, also known as volumetric rescanning or uniform repainting.
The problem of organ motion and rescanning synchronism has also been tackled by several groups, including Furukawa et al [45] and Seco et al [37]. Solutions include:
using random modulations, e.g. a change in scan speed
repainting energy slices in different orders (random repainting)
random delays between repaints (time delay)
change in scan paths between two rescans
use of data from motion monitoring systems in combination with modulation dose rate, either as phase-controlled rescanning or as breath-sampled rescanning.
Data from phase-controlled rescanning and breath-sampled rescanning show that uniform spreading of rescans over the motion of the breathing cycle leads to more robust treatment delivery, requiring fewer rescans than the other methods for the same level of homogeneity in the CTV. There are also two methods of delivering rescanned treatments [35]:
Scaled rescanning—this is delivering each rescan with a proportionally reduced dose per scan and is the most typical method.
Isolayered rescanning—where a specified number of protons per spot are delivered at each scan, which leads to a different spot position in each rescan. This is because some spots will have received sufficient dose from previously completed scans.
Beam tracking
With beam tracking [44], the motion of the CTV is monitored and compensated for so that a PTV margin is reduced. Owing to the need to compensate for both CTV motion and the change in radiological path length in proton therapy, the German national heavy-ion physics laboratory, the Gesellschaft für Schwerionen, in Darmstadt, has established a method of using a motor-driven compensation system for changes in radiological path length [46,47] and raster scanning [45,48].
Margins
In X-ray radiotherapy, the PTV is used to provide the safety margins for all uncertainties in planning and delivery [21,22]. The objective with current X-ray technology is to deliver a dose between +7% and −5% of the prescribed dose, with dose coverage which is as uniform as possible. Geometric uncertainties owing to positional discrepancy or motion are unavoidable. Random uncertainties act to “blur” the distribution while systematic uncertainties shift the entire dose distribution [49]. A shift in the cumulative dose distribution can result in part of the target being missed and potential overdose to normal tissue.
The widely used CTV–PTV margin recipe, derived by van Herk [50], is used to ensure that 90% of patients have CTV coverage of at least 95% of the prescribed dose. The static PTV represents the moving CTV and is therefore a useful method of evaluating a plan, by using DVHs to report the minimum dose to the CTV. In proton therapy, if conventional PTV margins do not produce plans robust to uncertainties, another method of ensuring confidence in a planned dose distribution representing the delivered dose distribution is required. This can be achieved by increasing margins or “smearing” the proton range with a compensator in the case of passive scattering. However, this will lead to a reduction in dose conformity in the plan. The concept of a safety margin also partly fails for set-up errors because they not only shift the dose distribution but also change the range where there are density changes in the beam path, leading to a distorted dose distribution [51]. In proton therapy, any concept used to compensate for organ motion-generated uncertainties must include both geometric motion and the influence it has on the beam range, as this can have a severe dosimetric impact [51].
PTV margin sizes in proton therapy have been investigated by Thomas [52]. In many comparative studies of achievable dose distributions between protons and X-rays, the CTV and PTV margin sizes are the same for both modalities. The CTV and OAR volumes are invariably the same for any treatment modality. However, the size of the margin between CTV to PTV and OAR to PRV is modality dependant. Thomas [52] quantified margins required for PTVs and PRVs in proton therapy for anterior single, anterior–posterior parallel opposed and four-field brick proton beam arrangements. Each type of systematic and random error was considered and the effect they had in geometry and range was discussed, as was the modality dependency. The key message is that isodoses defined by lateral edges, for instance, can be treated in the same way as for X-rays, whereas isodoses defined by the distal edge will have a different uncertainty arising from inaccurate electron densities derived from CT data. Margins were calculated for a head and neck (H&N) and a prostate case. For the H&N case the CTV–PTV margins in all three planes for the single field and parallel opposed fields were smaller than those required by X-rays; however, they were greater for the four-field brick plan. This same pattern was observed for the prostate case. This is because a smaller margin size is needed in the anterior–posterior direction for single and parallel opposed (3 mm compared with 10.5 mm for the H&N case), as set-up errors and motion in this direction will not affect the dose distribution.
Albertini et al [53] addressed the issue of whether safety margins in proton planning are necessary. In this paper two types of treatment delivery were investigated: single field uniform dose (SFUD) plans and IMPT plans. Plans included:
an SFUD plan to the PTV
an IMPT plan delivering uniform fields to the PTV
a non-uniform field IMPT plan, with strict constraints on OARs, planned to the PTV
a non-uniform field IMPT plan, with strict constraints on OARs, planned to the CTV.
The robustness of each plan to random set-up and systematic range uncertainties was compared. Robustness was determined using the concept of “error bar dose distributions”, by shifting dose distributions relative to the expected errors and then displaying a final “error bar” dose distribution and “error bar” volume histogram; therefore, it was representative of the possible discrepancies in dose between planning and delivery. The results from this work show that, for uniform field deliveries [(i) and (ii)], the use of margins improved the plan robustness, where <5% of the CTV contained errors >10%. However, in highly complex non-uniform IMPT plans [(iii) and (iv)], margins improved robustness only marginally and, for 5% of the CTV, errors of up to 55% were observed. For plans (iii) and (iv), steep dose gradients existed within the target, leading to uncertainties within the target. Because margins can help CTV coverage only at the edges of the target volume and not in the centre, they have little effect on plan robustness when steep dose gradients exist within the target volume [53]. It was found, however, that complex IMPT plans were robust to OARs when they were included in the optimisation as a constraint. It was concluded that there is a need for more sophisticated methods for taking into account uncertainties in highly modulated IMPT plans, such as including them in the optimisation. This work assumed that the set-up uncertainty was indeed random and considered only the systematic component of the range error. It did not include the effect on the dose distribution from motion uncertainties.
Optimisation functions for robust proton planning
An alternative to using margins is being developed by incorporating errors directly into the optimisation algorithm, as proposed by Unkelbach et al [54] and Pflugfelder et al [55]. This can be implemented because in IMPT there exist many solutions which are all dosimetrically equivalent. This “degeneracy” of solutions can be used to reduce the sensitivity of the plan to uncertainties if they are incorporated into the optimisation process [55].
Unkelbach et al [54] investigated methods for incorporating range uncertainties into the treatment planning process and simulated doses for a non-patient case. The two approaches taken were the probabilistic approach and robust linear programming. The first assumed prior knowledge of the probability distribution of the uncertainty; in most cases, the distribution was assumed normal. The latter optimised the worst case that could have occurred. The optimisation used by the Deutsches Krebsforschungszentrum (Heidelberg, Germany) in-house TPSs for particle therapy (KonRad, Heidelberg, Germany), is the worst-case dose distribution approach [55]. The worst-case dose distribution was introduced by Lomax et al [56] and is a method of combining multiple dose distributions into a single one. For a voxel inside the target volume the minimum dose to this voxel is stored and for a voxel outside of the target volume the maximum dose is stored. Each voxel is treated independently so the worst-case dose distribution is an “unphysical” one. Although the worst-case dose distribution is unphysical it can be used as a lower bound for the worst-quality treatment plan. A best-case plan can be seen as the upper bound in the same respect, but to the best achievable plan quality. In this optimisation it is assumed that the range uncertainties for each Bragg peak are correlated, so that at the target the range uncertainty is accumulated and effects within the target are ignored. The dose from each beamlet was calculated at multiple ranges; three ranges between 2 and 5 mm were used, and these were the nominal, maximum and minimum range uncertainties [56]. The set-up uncertainty was modelled as a shift of the target inside a sphere with a radius equal to the maximum set-up uncertainty. They concluded that both methods led to treatment plans less sensitive to range variations and that both plans were of a similar quality. This was achieved by using the lateral edge instead of the distal edge to shape the dose distribution at the transition between the OAR and the tumour for both methods.
Pflugfelder et al [55] applied the worst-case optimisation method to real patient data where the target surrounded the spinal cord. Uncertainties of the beamlet ranges of ±5 mm were considered. The range uncertainty was sampled at three positions: the nominal range, the maximum range and the minimum range. Using this optimisation process, the plan's sensitivity to range uncertainties was decreased. However, this was achieved by compromising the dosimetric quality of the plan by:
using the lateral instead of the distal edges of the Bragg peaks to shape the dose gradients between the target and the OAR
adding a “safety margin” automatically at the distal field edge for each treatment beam
flattening the dose profile in depth for each treatment beam compared with the nominal plan.
For the tumour, the largest deviations between delivered and prescribed doses corresponded to an underdosage close to the OAR, whereas the deviations in most other parts of the tumour were small. The maximum doses delivered to OAR voxels reached approximately 80% of the prescribed dose [55]. The DVHs from each method showed very similar results. This group [55] showed comparable results to those of Unkelbach et al [54] using their worst-case optimisation function; owing to the simplicity of the worst-case optimisation, it was expected to be faster [55]. When using the worst-case optimisation incorporating both set-up and range uncertainties, the resulting change to the dose distribution was such that the distal dose gradient of the treatment beam was smoothed.
Chen et al [57] introduced a method of including robustness into a multicriteria optimisation (MCO) framework for IMPT. In current inverse planning systems for IMRT the dose distribution is determined by a computerised optimisation based on dose prescriptions for targets and other volumes which have been assigned an importance level [58]. To determine the plan quality, a number is assigned based on the deviation from prescription dose in each volume and the optimisation result is the plan with the lowest number. This is a trial and error process, given that the resulting plan may not be clinically acceptable and the importance levels assigned to each volume may need adjusting and the optimisation rerun. This can be time consuming and the best-quality plan may not have been achieved as the planner cannot try every combination of parameters. There also exists a problem that, if upper and lower constraints are met, the optimisation process will not further improve doses to these volumes. This means that IMRT and IMPT cannot be exploited to their full potential owing to limitations with inverse planning [58]. The concept of multi-objective Pareto optimisation (often known as MCO) has been introduced into radiotherapy treatment planning to overcome these problems [59]. A Pareto optimal treatment plan is not a single plan but a database of plans where each one represents a Pareto optimal solution which cannot be improved without worsening at least one other parameter [58]. In this case the planner can navigate through the pre-calculated database of Pareto optimal plans and visualise in real time the trade-offs for each case [60]. MCO allows for the plan which strikes the best balance between different objectives to be selected from a Pareto front.
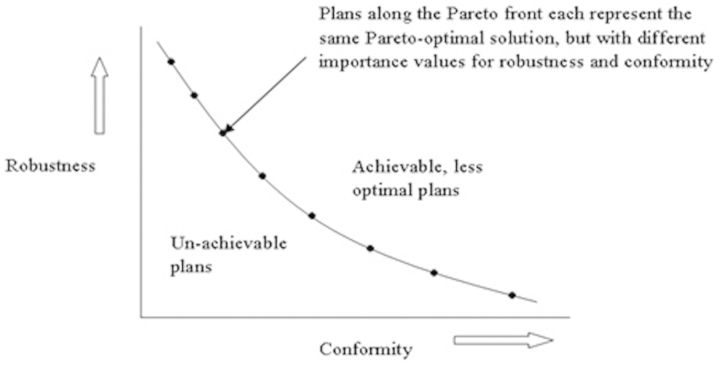
Chen et al [57] suggested that the trade-off between robustness and dosimetric quality in IMPT can be investigated using MCO. An example is shown in Figure 3. A planning objective for robustness represents the worst case realised for any error scenario and so can provide a measure of plan robustness. The MCO method used by this group was based on a linear projection solver using “minimax” optimisation, meaning that the objective was minimised for the worst-case error scenario and was used to investigate plan robustness to uncertainties. The computing time required for the robustness optimisation for each Pareto optimal plan was 5 min. Range uncertainties have been modelled using an overshoot and undershoot scenario of ±3%, as used by Lomax [28]. The set-up uncertainty was modelled using discrete rigid shifts of the patient with respect to the isocentre. A base of skull tumour was planned twice: once using robust optimisation (but without MCO) and once with margins. A chordoma case was planned with MCO as an ideal example of the trade-off between brain stem sparing, CTV coverage and robustness. In the skull base case the DVH of the CTV for both the robust plan and the margin plan showed similar coverage for the nominal plan. However, once plans with errors were introduced, the coverage was worse for the margin case. In consideration of brain stem sparing the robust plan ensured that in all error cases the dose was limited to 60 Gy; this was not seen in the margin plan. In the chordoma case the Pareto surface of objectives allowed the planner to have greater control when deciding between a robust plan, a conformal plan or somewhere in between.
Figure 3.
Illustrating two parameters for optimisation, conformity and robustness, and how a Pareto front can be used to investigate clinically achievable plans with the same Pareto-optimised solution, but with different values of importance on robustness and conformity.
Treatment analysis and validation
For all areas in radiotherapy, validation and quality assurance are routinely carried out. Phantom work and technical assessment are carried out when a new technology or method is being implemented, such as motion mitigation techniques. However, patient-specific quality assurance is also required, and this starts at the decision to accept a given plan design. In cases where margins are in use, the PTV is used as a tool for reporting the minimum dose to the CTV. If the PTV method is to be discarded then a new method for recording dose to a moving CTV, and for evaluating plans through the use of DVHs, is needed. As discussed above, Albertini et al [61] have devised a possible solution to this problem by determining plan robustness for set-up and range errors using an error bar dose distribution method. This method was later validated experimentally using a customised anthropomorphic phantom based on a diagnostic head phantom, and GafChromic® EBT2 batch F100070903B film, to carry out patient-specific quality assurance under realistic conditions and with deliberately introduced errors. A large part of ensuring optimum treatment delivery to the patient is verifying the accuracy of the dose delivered and comparing it with that predicted by the planning system. In cases where uniform fields are being delivered it is enough to experimentally measure the individual field doses, and in the case of proton therapy, the dose in homogeneous water [61]. The highly modulated nature of IMPT means that ever more complex methods of plan verification are being developed to cope with the advancing treatment technology to ensure safe patient treatment. Safai et al [62] described how a scintillation dosimetry system was used to verify such proton treatments. The problem with this method was that, even though it could accurately measure the dose delivered, the dose from the TPS needed to be recalculated in a homogeneous material to be compared with the measured results [61]. This approach of patient-specific plan verification is not adequate when fields of inhomogeneous dose distributions are being applied. Each inhomogeneous dose distribution will, in combination, achieve a uniform dose in the target volume, but any slight misalignment of a steep dose gradient could lead to a severe under- or overdosage in comparison with the plan [61]. This effect could be worsened by the presence of density heterogeneities in the patient [61]. By using the customised phantom, Albertini et al [61] have been able to measure the accuracy of dose and the effect of spatial and range errors by deliberately introducing errors. Set-up errors were introduced by moving the phantom by known amounts, and range errors simulated by modifying the HU values by ±3% [19]. From this work it was found that 3D IMPT plans were more robust than DET plans. The theory is that there are fewer Bragg peaks used in DET, whereas in 3D IMPT more spots are used (∼180 for DET compared with ∼1500 for 3D IMPT in the example used), each with a lower weighting than those in DET, so that that any misalignments would have a greater impact on the DET plan.
Discussion
To produce optimal plans using protons, knowledge of the proton range in the patient is essential. There are many factors that can contribute to range uncertainty. HU uncertainties can contribute to approximately ±3% uncertainty in range even after site-specific CT scanner calibrations have been carried out. Owing to the steep dose gradients that can be achieved at the edges of, and within, the target volume, precise field matching is required to prevent over- or underdosage in the target. Simulation and patient data examples have been used to show that the use of a PTV margin does not ensure uniform dose coverage to the CTV for complex IMPT plans. Despite these results, there is still a need for facility-based and treatment protocol-specific simulations to be carried out to achieve quantitative assessments for different tumour sites [44]. Parameters such as dose regime, scan path pattern and beam extraction rate need to be included because they will affect the resulting dose distribution.
Methods of improving plan robustness to range uncertainties beyond the use of margins are being developed for use in complex IMPT plans to ensure CTV coverage. However, all of these methods will decrease the achievable conformity of a plan. Robust optimisation using the worst-case scenario will produce a robust plan, sacrificing conformity by placing a lateral edge instead of the sharp distal edge to shape the dose between the target and the OAR. The use of robust MCO gives the planner greater control over objective weights. This is important because there is little evidence or known experience in determining what type of plan, conformal or robust, is most beneficial for the population of patients.
The difficulty with deliberately introducing uncertainties into either the optimisation or the plan evaluation is that all probability distributions are assumed to be known. Albertini et al [53] and Chen et al [57] only considered range uncertainties created in the range calculation arm in Figure 2. The assumption is then that any change in radiological path length caused by set-up or motion, whether during a fraction or between fractions, has a negligible effect on the range. Motion errors will be dependent on anatomical location and set-up error will be protocol specific, based on what image guided radiotherapy treatment (IGRT) schedules are in place and the type of immobilisation used. More research is needed in quantifying range uncertainties for different anatomical locations so that they can be implemented into the robust optimisation of dose modelling. This would include investigating range errors associated with different IGRT schemes, types of motion, and when to use adaptive planning.
In the absence of a PTV, novel methods to evaluate dose coverage have been developed such as the error bar DVH and the use of worst-case and best-case optimisation to give upper and lower bounds on a DVH. A challenge for all these methods will be how to carry out adequate and efficient patient-specific verification. Currently, studies on beam tracking and rescanning have not been carried out using patient data. The data available from non-patient heterogeneity studies have shown that rescanning techniques produce improved results compared with beam tracking, but further investigations using real patient data are required [44]. There is no information in the literature regarding patient-specific validation for rescanning and beam tracking methods because they are not yet in routine clinical use.
Adaptive radiotherapy is the adjustment during the treatment course of the parameters initially chosen at planning, in order to re-optimise the treatment as a direct result of unavoidable changes in the patient. In proton therapy, the use of adaptive therapy may prove to be valuable for ensuring the delivered dose matches the planned dose at each fraction. In this context, access to high-quality daily volumetric imaging, and fast-dose recalculation algorithms and computing power, will be required. This may provide the opportunity for greater individualisation of the patient's treatment. Use of a multi-objective Pareto optimisation function could allow plans to be recalculated when necessary using different weights for robustness and conformity, incorporating image guidance data to optimise the plan.
A significant advantage of conventional X-ray therapy over proton therapy is the wealth of experience and knowledge available. A key area for optimising the treatment planning process is in gaining experience in planning proton treatments. The number of proton facilities available worldwide is rapidly increasing, yet there is a substantial shortage of oncologists, dosimetrists and physicists with the required expertise [63]. In the UK, patients have been able to access proton therapy abroad under the auspices of the National Health Service Proton Overseas Programme since 2008 [64], and the UK government recently announced that two proton centres will be established in England. It is hoped that these will start to treat patients in the next 4–5 years [65,66], such that developments discussed here will be directly relevant.
It has been shown that a PTV may not be the best solution for ensuring target coverage in the case of complex IMPT plans. This is the result of the sensitivity of the proton range to density heterogeneities and the achievable steep dose gradients inside the volume. Many groups have been working towards possible solutions for achieving dosimetric quality and plan evaluation without a PTV. The solutions described here show great promise for optimising proton therapy planning.
Acknowledgments
We are grateful to Dr Simon Thomas for helpful discussions.
Footnotes
SEM's PhD is funded by the Medical Research Council. NGB is supported by the National Institute for Health Research (NIHR), Cambridge Biomedical Research Centre.
References
- 1.Bortfeld T. Optimized planning using physical objectives and constraints. Semin Radat Oncol 1999;9:20–34. [DOI] [PubMed] [Google Scholar]
- 2.Bragg WH. On absorption of alpha rays and on the classification of the alpha rays from radium. Phil Mag 1904;6:719–25. [Google Scholar]
- 3.Merchant TE, Hua CH, Shukla H, Ying X, Nill S, Oelfke U. Proton versus photon radiotherapy for common pediatric brain tumors: comparison of models of dose characteristics and their relationship to cognitive function. Pediatr Blood Cancer 2008;51:110–17. [DOI] [PubMed] [Google Scholar]
- 4.Ares C, Hug EB, Lomax AJ, Bolsi A, Timmermann B, Rutz HP, et al. Effectiveness and safety of spot scanning proton radiation therapy for chordomas and chondrosarcomas of the skull base: first long-term report. Int J Radiat Oncol Biol Phys 2009;75:1111–18. [DOI] [PubMed] [Google Scholar]
- 5.De Ruysscher D, Lodge M, Jones B, Brada M, Munro A, Jefferson T, et al. Charged particles in radiotherapy: a 5-year update of a systematic review. Radiother Oncol 2012;103:5–7. [DOI] [PubMed] [Google Scholar]
- 6.Allen AM, Pawlicki T, Dong L, Fourkal E, Buyyounouski M, Cengel K, et al. An evidence based review of proton beam therapy: the report of ASTRO's emerging technology committee. Radiother Oncol 2012;103:8–11. [DOI] [PubMed] [Google Scholar]
- 7.Jones B. The potential clinical advantages of charged particle radiotherapy using protons or light ions. Clin Oncol (R Coll Radiol) 2008;20:555–63. [DOI] [PubMed] [Google Scholar]
- 8.Durante M, Loeffler JS. Charged particles in radiation oncology. Nature 2010;7:37–43. [DOI] [PubMed] [Google Scholar]
- 9.Lodge M, Pijls-Johannesma M, Stirk L, Munro AJ, De Ruysscher D, Jefferson T. A systematic literature review of the clinical and cost-effectiveness of hadron therapy in cancer. Radiother Oncol 2007;83:110–22. [DOI] [PubMed] [Google Scholar]
- 10.van deWater T, Bijl HP, Schilstra C, Pijls-Johannesma M, Langendijk J. The potential benefit of radiotherapy with protons in head and neck cancer with respect to normal tissue sparing: a systematic review of literature. Oncologist 2011;16:366–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramaekers BL, Pijls-Johannesma M, Joore MA, van denEnde P, Langendijk JA, Lambin P, et al. Systematic review and meta-analysis of radiotherapy in various head and neck cancers: comparing photons, carbon-ions and protons. Cancer Treat Rev 2011;37:185–201. [DOI] [PubMed] [Google Scholar]
- 12.Haberer T, Becher W, Schardt D, Kraft G. Magnetic scanning system for heavy ion therapy. Nucl Instrum Methods Phys Res A 1993;330:296–305. [Google Scholar]
- 13.Pedroni E, Bacher R, Blattmann H, Böhringer T, Coray A, Lomax A, et al. The 200-MeV proton therapy project at the Paul Scherrer Institute: conceptual design and practical realization. Med Phys 1995;22:37–53. [DOI] [PubMed] [Google Scholar]
- 14.DeLaney TF, Hanne MK. Proton and charged particle radiotherapy. Philadelphia, PA: Lippincott Williams and Wilkins; 2009. [Google Scholar]
- 15.Lomax A. Intensity modulation methods for proton radiotherapy. Phys Med Biol 1999;44:185–205. [DOI] [PubMed] [Google Scholar]
- 16.Deasy J. A proton dose calculation algorithm for conformal therapy simulations based on Moliere theory of lateral deflections. Med Phys 1998;25:476–83. [DOI] [PubMed] [Google Scholar]
- 17.Oelfke U, Bortfeld T. Intensity modulated radiotherapy with charged particle beams: studies of inverse treatment planning for rotation therapy. Med Phys 2000;27:1246–57. [DOI] [PubMed] [Google Scholar]
- 18.Nill S, Bortfeld T, Oelfke U. Inverse planning of intensity modulated proton therapy. Z Med Phys 2004;14:35–40. [DOI] [PubMed] [Google Scholar]
- 19.Lomax A. Intensity modulated proton therapy and its sensitivity to treatment uncertainties 2: the potential effects of inter-fraction and inter-field motions. Phys Med Biol 2008;53:1043–56. [DOI] [PubMed] [Google Scholar]
- 20.España S, Paganetti H. The impact of uncertainties in the CT conversion algorithm when predicting proton beam ranges in patients from dose and PET-activity distributions. Phys Med Biol 2010;55:7557–71. [DOI] [PubMed] [Google Scholar]
- 21.International Commission on Radiation Units and Measurements: Prescribing, recording, and reporting photon beam therapy. ICRU Report no. 50. Bethesda, MD: ICRU; 1993. [Google Scholar]
- 22.International Commission on Radiation Units and Measurements: A review of the new supplement to ICRU Report 50. ICRU Report no. 62. Bethesda, MD: ICRU; 1999. [Google Scholar]
- 23.International Commission on Radiation Units and Measurements: Prescribing, recording, and reporting proton beam therapy. ICRU Report no. 78. Bethesda, MA: ICRU; 2007. [Google Scholar]
- 24.Chvetsov AV, Paige SL. The influence of CT image noise on proton range calculation in radiotherapy planning. Phys Med Biol 2010;55:N141–9. [DOI] [PubMed] [Google Scholar]
- 25.Schaffner B, Pedroni E. The precision of proton range calculations in proton radiotherapy treatment planning: experimental verification of the relationship between CT-HU and proton stopping power. Phys Med Biol 1998;43:1579–92. [DOI] [PubMed] [Google Scholar]
- 26.Schulte R, Bashkirov V, Li T, Liang Z, Mueller K, Heimann J, et al. Conceptual design of a proton computed tomography system for applications in proton therapy. IEEE Trans Nucl Sci 2004;51:866–72. [Google Scholar]
- 27.Moyers MF, Sardesai M, Sun S, Miller DW. Ion stopping powers and CT numbers. Med Dosim 2010;35:179–94. [DOI] [PubMed] [Google Scholar]
- 28.Lomax A. Intensity modulated proton therapy and its sensitivity to treatment uncertainties 1: the potential effects of calculational uncertainties. Phys Med Biol 2008;53:1027–42. [DOI] [PubMed] [Google Scholar]
- 29.Burnet NG, Adams EJ, Fairfoul J, Tudor GS, Hoole AC, Routsis DS, et al. Practical aspects of implementation of helical tomotherapy for intensity-modulated and image-guided radiotherapy. Clin Oncol 2010;22:294–312. [DOI] [PubMed] [Google Scholar]
- 30.Booth JT, Zavgorodni SF. Set-up error and organ motion uncertainty: a review. Australas Phys Eng Sci Med 1999;22:29–47. [PubMed] [Google Scholar]
- 31.Langen KM, Jones DT. Organ motion and its management. Int J Radiat Oncol Biol Phys 2001;50:265–78. [DOI] [PubMed] [Google Scholar]
- 32.Rimmer YL, Burnet NG, Routsis DS, Twyman N, Hoole A, Treeby J, et al. Practical issues in the implementation of image-guided radiotherapy for the treatment of prostate cancer within a UK department. Clin Oncol 2008;20:22–30. [DOI] [PubMed] [Google Scholar]
- 33.Lambert J, Suchowerska N, McKenzie DR, Jackson M. Intrafractional motion during proton beam scanning. Phys Med Biol 2005;50:4853–62. [DOI] [PubMed] [Google Scholar]
- 34.Phillips MH, Pedroni E, Blattmann H, Boehringer T, Coray A, Scheib S. Effects of respiratory motion on dose uniformity with a charged particle scanning method. Phys Med Biol 1992;37:223–34. [DOI] [PubMed] [Google Scholar]
- 35.Zenklusen SM, Pedroni E, Meer D. A study on repainting strategies for treating moving targets with proton pencil beam scanning at the new Gantry 2 at PSI. Phys Med Biol 2010;55:5103–21. [DOI] [PubMed] [Google Scholar]
- 36.Knopf AC, Hong TS, Lomax AJ. Scanned proton radiotherapy for mobile targets—the effectiveness of re-scanning in the context of different treatment planning approaches and for different motion characteristics. Phys Med Biol 2011;56:7257–71. [DOI] [PubMed] [Google Scholar]
- 37.Seco J, Robertson D, Trofimov A, Paganetti H. Breathing interplay effects during proton beam scanning: simulation and statistical analysis. Phys Med Biol 2009;54:N283–94. [DOI] [PubMed] [Google Scholar]
- 38.Grözinger SO, Rietzel E, Li Q, Bert C, Haberer T, Kraft G. Simulations to design an online motion compensation system for scanned particle beams. Phys Med Biol 2006;51:3517–31. [DOI] [PubMed] [Google Scholar]
- 39.Bert C, Grözinger SO, Rietzel E. Quantification of interplay effects of scanned particle beams and moving targets. Phys Med Biol 2008;53:2253–65. [DOI] [PubMed] [Google Scholar]
- 40.Carabe A, Moteabbed M, Depauw N, Schuemann J, Paganetti H. Range uncertainty in proton therapy due to variable biological effectiveness. Phys Med Biol 2012;57:1159–72. [DOI] [PubMed] [Google Scholar]
- 41.Robertson J, Williams J, Schimdt R, Little J, Flynn D, Suit H. Radiobiological studies of high energy modulated proton beam utilizing cultured mammalian cells. Cancer 1975;35:1664–77. [DOI] [PubMed] [Google Scholar]
- 42.Matsuura T, Egashira Y, Nishio T, Matsumoto Y, Wada M, Koike S, et al. Apparent absence of a proton beam dose rate effect and possible differences in RBE between Bragg peak and plateau. Med Phys 2010;37:5376. [DOI] [PubMed] [Google Scholar]
- 43.Grassberger C, Trofimov A, Lomax A, Paganetti H. Variations in linear energy transfer within clinical proton therapy fields and the potential for biological treatment planning. Int J Radiat Oncol Biol Phys 2011;80:1559–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bert C, Durante M. Motion in radiotherapy: particle therapy. Phys Med Biol 2011;56:R113–44. [DOI] [PubMed] [Google Scholar]
- 45.Furukawa T, Inaniwa T, Sato S, Tomitani T, Minohara S, Noda K, et al. Design study of a raster scanning system for moving target irradiation in heavy-ion radiotherapy. Med Phys 2007;34:1085–97. [DOI] [PubMed] [Google Scholar]
- 46.Bert C, Saito N, Schmidt A, Chaudhri N, Schardt D, Rietzel E. Target motion tracking with a scanned particle beam. Med Phys 2007;34:4768–71. [DOI] [PubMed] [Google Scholar]
- 47.Saito N, Bert C, Chaudhri N, Gemmel A, Schardt D, Durante M, et al. Speed and accuracy of a beam tracking system for treatment of moving targets with scanned ion beams. Phys Med Biol 2009;54:4849–62. [DOI] [PubMed] [Google Scholar]
- 48.Keall PJ, Kini VR, Vedam SS, Mohan R. Motion adaptive x-ray therapy: a feasibility study. Phys Med Biol 2001;46:1–10. [DOI] [PubMed] [Google Scholar]
- 49.Grözinger SO, Li Q, Rietzel E, Haberer T, Kraft G. 3D online compensation of target motion with scanned particle beam. Radiother Oncol 2004;73 (Suppl. 2)S77–9. [DOI] [PubMed] [Google Scholar]
- 50.van Herk M. Errors and margins in radiotherapy. Semin Radiat Oncol 2004;14:52–64. [DOI] [PubMed] [Google Scholar]
- 51.Grözinger SO, Bert C, Haberer T, Kraft G, Rietzel E. Motion compensation with a scanned ion beam: a technical feasibility study. Radiat Oncol 2008;3:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thomas SJ. Margins for treatment planning of proton therapy. Phys Med Biol 2006;51:1491. [DOI] [PubMed] [Google Scholar]
- 53.Albertini F, Hug EB, Lomax AJ. Is it necessary to plan with safety margins for actively scanned proton therapy? Phys Med Biol 2011;56:4399. [DOI] [PubMed] [Google Scholar]
- 54.Unkelbach J, Chan T, Bortfield T. Accounting for range uncertainties in the optimisation of intensity modulated proton therapy. Phys Med Biol 2007;52:2755–73. [DOI] [PubMed] [Google Scholar]
- 55.Pflugfelder D, Wilkens JJ, Oelfke U. Worst case optimization: a method to account for uncertainties in the optimization of intensity modulated proton therapy. Phys Med Biol 2008;53:1689–700. [DOI] [PubMed] [Google Scholar]
- 56.Lomax AJ, Pedroni E, Rutz H, Goitein G. The clinical potential of intensity modulated proton therapy. Z Med Phys 2004;14:147–52. [DOI] [PubMed] [Google Scholar]
- 57.Chen W, Unkelbach J, Tromfimov A, Madden T, Kooy H, Bortfeld T. Including robustness in multi-criteria optimization for intensity-modulated proton therapy. Phys Med Biol 2012;57:591–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thieke C, Küfer K, Monz M, Scherrer A, Alonso F, Oelfke U, et al. A new concept for interactive radiotherapy planning with multicriteria optimization: first clinical evaluation. Radiother Oncol 2007;85:292–8. [DOI] [PubMed] [Google Scholar]
- 59.Cotrutz C, Lahanas M, Kappas C, Baltas D. A multiobjective gradient-based dose optimization algorithm for external beam conformal radiotherapy. Phys Med Biol 2001;46:2161–75. [DOI] [PubMed] [Google Scholar]
- 60.Craft D, Halabi T, Bortfeld T. Exploration of tradeoffs in intensity-modulated radiotherapy. Phys Med Biol 2005;50:5857–68. [DOI] [PubMed] [Google Scholar]
- 61.Albertini F, Casiraghi M, Lorentini S, Rombi B, Lomax AJ. Experimental verification of IMPT treatment plans in an anthropomorphic phantom in the presence of delivery uncertainties. Phys Med Biol 2011;56:4415. [DOI] [PubMed] [Google Scholar]
- 62.Safai S, Shixiong L, Pedroni E. Development of an inorganic scintillating mixture for proton beam verification dosimetry. Phys Med Biol 2004;49:4637–55. [DOI] [PubMed] [Google Scholar]
- 63.Dosanjh M. Development of hadron therapy for cancer treatment in Europe. AIP Conf Proc 2008;1032:12–16. [Google Scholar]
- 64.www.nhs.uk [homepage on the internet]. London, UK: NHS Specialised Services; 2011. [cited May 2011]. Available from: www.specialisedservices.nhs.uk/serv/proton-beam-therapy. [Google Scholar]
- 65.www.dh.gov.uk [homepage on the internet]. London, UK: Department of Health; 2012. [cited May 2011]. Available from: mediacentre.dh.gov.uk/2012/04/05/centres-selected-to-host-cutting-edge-cancer-services/ [Google Scholar]
- 66.Burnet N, Taylor E, Kirkby K, Thorp N, Mackay R, McKenna G. Proton beam therapy for cancer: an important development for patients with an explicit agenda. BMJ 2012;344:e2488.22511301 [Google Scholar]