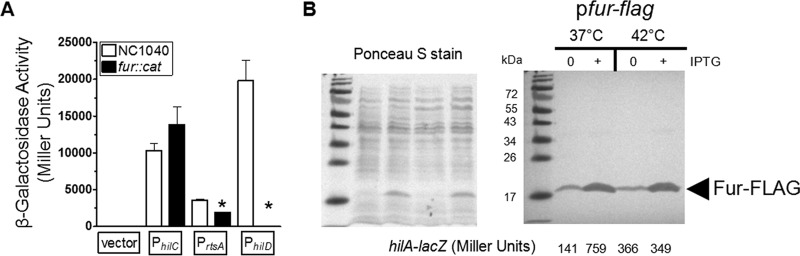
FIG 4.
Growth at 42°C repressed expression of SPI-1 genes independently of the activator Fur. (A) The promoters of hilC, rtsA, and hilD were cloned into the pSP417 multicopy shuttle vector (empty vector) upstream of a promoterless lacZ gene. Bacteria were grown as described for Fig. 1B, and the promoter activities were determined for the NC1040 and fur::cat strains following overnight growth at 37°C. Data are from 3 separate experiments. The strains used were BTNC0002 to BTNC0005 and BTNC0006 to BTNC0009. (B) Expression of a hilA-lacZ fusion at the chromosomal att site was determined following overnight growth with or without induction of the Fur-FLAG protein. Bacteria were grown as described for Fig. 1B, except that samples were diluted 500-fold and cultivated at either 37°C or 42°C. A portion of each sample was removed to measure β-galactosidase activity, and the remainder was treated for SDS-PAGE and Western blotting to detect the FLAG epitope. Following transfer of proteins to the nitrocellulose membrane, the membrane was stained with Ponceau S to ensure that equivalent levels of protein were loaded for samples and that ∼2 × 108 cells were loaded per lane (left panel). IPTG was added to the growth medium to reach a concentration of 0.1 mM to induce Fur-FLAG. Western blotting with the anti-FLAG antibody revealed cross-reactivity to the Fur protein of the expected size, indicated by the arrowhead with the appropriate label (right panel). The β-galactosidase activity for each sample is listed below each lane (right panel). Samples shown are representative of the results of 3 separate experiments. The complete β-galactosidase activity data are listed here. For BTNC0017 (pfur-flag), the values measured at 37°C were 156 ± 33 under uninduced conditions and 657 ± 89 under induced conditions and the values measured at 42°C were 316 ± 70 under uninduced conditions and 345 ± 4 under induced conditions.