Abstract
Indole, a bacterial product of tryptophan degradation, has a variety of important applications in the pharmaceutical industry and is a biomarker in biological and clinical specimens. Yet, specific assays to quantitate indole are complex and require expensive equipment and a high level of training. Thus, indole in biological samples is often estimated using the simple and rapid Kovács assay, which nonspecifically detects a variety of commonly occurring indole analogs. We demonstrate here a sensitive, specific, and rapid method for measuring indole in complex biological samples using a specific reaction between unsubstituted indole and hydroxylamine. We compared the hydroxylamine-based indole assay (HIA) to the Kovács assay and confirmed that the two assays are capable of detecting microgram amounts of indole. However, the HIA is specific to indole and does not detect other naturally occurring indole analogs. We further demonstrated the utility of the HIA in measuring indole levels in clinically relevant biological materials, such as fecal samples and bacterial cultures. Mean and median fecal indole concentrations from 53 healthy adults were 2.59 mM and 2.73 mM, respectively, but varied widely (0.30 mM to 6.64 mM) among individuals. We also determined that enterotoxigenic Escherichia coli strain H10407 produces 3.3 ± 0.22 mM indole during a 24-h period in the presence of 5 mM tryptophan. The sensitive and specific HIA should be of value in a variety of settings, such as the evaluation of various clinical samples and the study of indole-producing bacterial species in the gut microbiota.
INTRODUCTION
Indole is widely distributed in the environment and is a component of diverse important compounds that occur in nature. In the pharmaceutical industry, synthesized indoles and their modified derivatives are popularly known for their medicinal properties. Indole analogs are significant components of a number of products, including vitamin supplements, dye, over-the-counter drugs, flavor enhancers, and perfumery. They are also used in the agricultural and plastics industries. Indole has been shown to play a role in regulating bacterial biofilm formation and virulence and influences diverse physiological processes, including host immune response (1–7).
Indole is produced by about 85 bacterial species, including Gram-positive and Gram-negative bacteria, through the enzymatic degradation of tryptophan (8). Once produced, indole can be chemically modified within the same bacterial cell or taken up and modified by non-indole-producing bacteria. The most common naturally occurring indole analog is 3-methylindole (skatole), although other analogs, such as indoxyl sulfate and indole-3-propionic acid, can be found (9–11).
Indole production by bacteria is an important phenotypic characteristic that has long been used to differentiate, identify, and diagnose enteric bacterial infections (12). Currently, the Kovács assay (13–17) is the most widely used method for detecting indole-producing bacteria. However, the key component, para-dimethylaminobenzaldehyde, reacts with a wide variety of indole-containing compounds (18–25). As a result of this nonspecificity, the Kovács assay cannot be used to reliably quantitate indole in complex biological samples. Furthermore, the Kovács assay produces high background interference (absorbance) when samples containing commonly used bacterial culture media, such as brain heart infusion (BHI), Luria-Bertani, and tryptone media, are tested. The specific measurement of indole includes gas chromatography or high-performance liquid chromatography (HPLC)-based purification in tandem with mass spectrometry analysis, techniques which are expensive and labor-intensive and may not be readily available. Thus, the current assay methods for measuring indole are either specific but difficult (gas chromatography, HPLC, and mass spectrometry) or nonspecific and relatively simple (Kovács assay). As a result, a simple and rapid assay to specifically quantitate indole, especially in biological samples, is needed. Here, we describe such an assay for quantitating indole in complex biological samples that is compatible with the most commonly used growth media and produces very low background absorbance.
MATERIALS AND METHODS
Reagents.
The Kovács reagent, hydroxylamine-HCl, indole, 1-methylindole, 2-methylindole, 3-methylindole (skatole), 3-indoleacetic acid, and indoxyl sulfate potassium salt were purchased from Sigma-Aldrich (St. Louis, MO). Other indole analogs tested were indole-3-propionic acid (Chem-Impex International Inc., Wood Dale, IL) and indoxyl acetate (Thermo Fisher Scientific, Waltham, MA). Bacterial stock (enterotoxigenic Escherichia coli ATCC 35401, strain H10407) was purchased from the American Type Culture Collection (Manassas, VA).
Hydroxylamine-based indole assay (HIA).
Freshly prepared indole standards ranging from 0 to 300 μM were prepared in 70% ethanol. Using a microtiter plate, indole standards or unknowns in a total volume of 100 μl were incubated for 15 min at room temperature with 25 μl of 5.3 M NaOH and 50 μl of 0.3 M hydroxylamine hydrochloride (NH2OH-HCl). Following incubation, 125 μl of 2.7 M H2SO4 was added, thoroughly mixed, and incubated at room temperature for up to 30 min to yield a pink solution that was measured spectrophotometrically. A spectral analysis of the colored product determined the optimum wavelength to be 530 nm. All measurements were made using the SpectraMax i3 spectrophotometer (Molecular Devices, Sunnyvale, CA).
Kovács assay.
The Kovács assay was based on previous publications (13–16) and modified using 100 μl of the above-described indole standards in 70% ethanol or samples of unknown indole concentrations. The samples were incubated with 150 μl of Kovács reagent (Sigma-Aldrich, St. Louis, MO) for up to 30 min at room temperature. The reaction produced a soluble product, which was measured spectrophotometrically at 530 nm.
In the HIA and Kovács assays, at least six known indole concentrations from 0 to 300 μM were tested in triplicate on each day of testing, and the mean results were used to construct a standard curve. Indole levels in unknowns (also tested in triplicate) were calculated by comparison of absorbance values to those of a standard curve run in the same experiment. Data were expressed in micrograms per milliliter or converted to micromolar concentrations using the molecular weight of indole (117.15 g/mol).
Indole levels in stools of healthy adults.
Stool samples were selected from subjects enrolled in the Cryptosporidium volunteer study, which was approved by the Committee for the Protection of Human Subjects, The University of Texas Health Science Center at Houston. Results of these studies have been reported elsewhere (26–28). Briefly, all subjects underwent a thorough medical examination and were found to be healthy and immunocompetent. Informed consent was obtained, and volunteers were challenged with one of several Cryptosporidium isolates. All stool samples used in the present study were collected prior to challenge or within 2 days postchallenge. Approximately 52.8% of the samples selected were collected before volunteers were exposed, and another 41.5% were collected within 24 h of exposure. Only three samples (5.7%) were from day 2 following challenge. Upon arrival in the laboratory, each fecal sample was placed in a vial and stored frozen at −80°C until use. Samples were then thawed and immediately prepared and tested for indole concentration.
For each sample, 250 mg of stool was weighed, placed in 2-ml microcentrifuge tubes, and suspended in 750 μl of 70% ethanol. The samples were vortexed at maximum speed for 30 s and incubated in a water bath at 70°C for 10 min. The tubes were vortexed again at maximum speed for 30 s and centrifuged at 14,000 rpm for 20 min at 40°C. Stool supernatants were then tested as described above.
Measurement of indole concentrations in bacterial cultures.
Single colonies of enterotoxigenic Escherichia coli strain H10407 were picked from BHI agar plates and inoculated into 5 ml of BHI broth supplemented with 5 mM l-tryptophan. The culture was incubated aerobically in a shaker at 250 rpm for 24 h (the optical density [OD] at 600 nm of the culture was 1.6). For the indole tests, 1 ml of the 5-ml culture was centrifuged at 15,000 rpm for 15 min, and 100 μl of the supernatant was tested in triplicate using the HIA and the Kovács assay as described above. The indole standards were prepared in brain heart infusion medium.
RESULTS
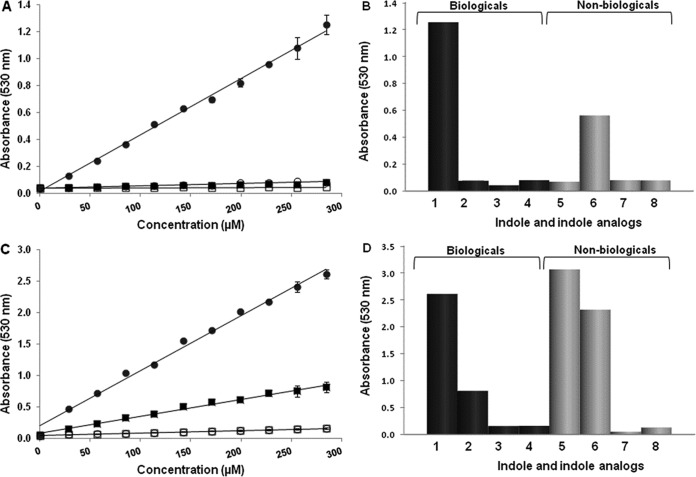
We exploited the property of hydroxylamine to specifically react with unsubstituted indole in order to develop a simple and specific assay for quantitating indole in biological samples. In this assay, indole standards and biological samples were incubated directly with hydroxylamine under basic conditions. Following the incubation period, the samples were acidified to produce a colored product that was read either visually or spectrophotometrically at 530 nm. The intensity of the color produced was directly proportional to the concentration of indole present (Fig. 1).
FIG 1.
The hydroxylamine-based indole assay (HIA) does not detect other naturally occurring indole analogs. Indole and indole analogs were tested in the hydroxylamine-based (A, B) and Kovács (C, D) assays. Each point (A, C) or bar (B, D) represents the mean value of four different experiments, each done in triplicate. The results of the HIA (A) and the Kovács assay (C) of naturally occurring (biological) indole (filled circles) and indole analogs (3-methylindole, filled squares; indole sulfate, open circles; indoxyl-3-proprionic acid, open squares) are shown for concentrations of 0 to 300 μM. Error bars indicate the standard deviations among the four replicate experiments. The HIA (B) and the Kovács assay (D) show absorbances of the following biological and nonbiological compounds at a concentration of 300 μM: indole (bar 1), 3-methylindole (bar 2), indoxyl-3-proprionic acid (bar 3), indole sulfate (bar 4), 2-methylindole (bar 5), 1-methylindole (bar 6), 3-indoleacetic acid (bar 7), and indoxyl acetate (bar 8).
Sensitivity and specificity of the HIA.
The HIA and Kovács assay were compared for sensitivity and reproducibility by using known concentrations of indole (Fig. 1). The two assays detected indole levels at micromolar concentrations and had a high degree of linearity (R2, >0.99) over all of the concentrations tested. The coefficient of variation was <10% for all absorbance values in both assays. These results showed that the HIA compares favorably to the Kovács assay in that both were reproducible and were able to detect microgram amounts of indole.
The specificities of the assays were compared by testing the three most common, naturally occurring indole analogs present in biological samples: 3-methylindole (skatole), indoxyl sulfate, and indole-3-propionic acid (9–11). The Kovács assay detected each of the three compounds, whereas the HIA detected only indole and did not yield appreciable values for any of the other three compounds at the concentrations tested (Fig. 1). The specificity of the HIA and Kovács assay were further assessed by testing synthetic indole-containing compounds, including 1-methylindole, 2-methylindole, 3-indoleacetic acid, and indoxyl acetate. It should be noted, however, that all of these compounds are synthetically produced indole derivatives, and none of these synthetic compounds occur naturally in tissues or any biological material. The Kovács assay readily detected 1-methylindole and 2-methylindole, while the HIA detected only 1-methylindole. Neither assay detected 3-indoleacetic acid or indoxyl acetate to any appreciable degree. Taken together, these results demonstrate that the two assays were comparable in terms of their reproducibility and sensitivity in detecting indole but that only the HIA was specific for indole under the described conditions. Thus, HIA can more accurately measure indole levels in biological materials without interference from naturally occurring indole analogs.
Indole measurement in stools of healthy adults and bacterial cultures.
Fecal samples were collected from healthy volunteers who had no clinical or microbiological evidence of either bacterial or parasitic infection. The HIA was optimized for the amount and volume of fecal sample needed for rapid quantitation of indole (data not shown). The mean age for all 53 subjects was 31.8 years, with a range of 21 to 50 years. Fecal indole levels among individuals varied widely from 0.30 mM to 6.64 mM, and the mean and median indole levels were 2.59 mM and 2.73 mM, respectively. The distribution of fecal indole levels resembled a normal curve, with the highest frequency (28.3%) in the 2.0 to 3.0 mM range (Fig. 2). Furthermore, the great majority (75%) of fecal indole concentrations fell within the range of 1.0 to 4.0 mM.
FIG 2.

Frequency distribution of fecal indole levels (in micromolar) in 53 healthy adults as determined by the hydroxylamine-based assay. Each sample was assayed in duplicate, and mean values are expressed as micromolar concentrations. The percentage of subjects in each category is shown.
To demonstrate whether the assay can be used to quantitate indole levels in bacterial culture media, single colonies of enterotoxigenic E. coli strain H10407 were inoculated in BHI broth supplemented with 5 mM tryptophan. Following 24 h of incubation at 37°C, the supernatants were tested using the HIA and the Kovács assay. Background absorbances with culture medium alone (i.e., a 0.327 OD at 530 nm for the Kovács assay and a 0.044 OD at 530 nm for the HIA) were subtracted from E. coli culture results. The HIA detected 3.3 ± 0.22 mM in the bacterial culture, compared to the 4.7 ± 0.33 mM detected by the Kovács assay (Fig. 3).
FIG 3.
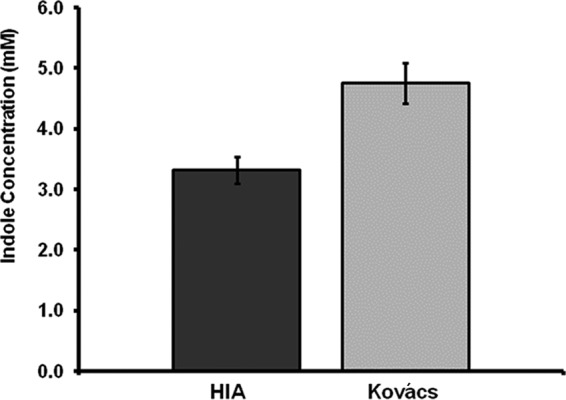
Comparative quantitation of indole concentrations in bacterial cultures. Enterotoxigenic E. coli strain H10407 was grown for 24 h, and supernatants were tested using the hydroxylamine-based indole assay (HIA) or the Kovács assay. Data represent mean values ± standard deviations from three separate experiments, each done in triplicate.
DISCUSSION
Indole is an important bacterial product involved in many biological processes and is used in a variety of pharmaceutical and industrial systems. As a result, there is a new interest in harnessing the inherent beneficial properties of indole to improve human health. Furthermore, due to the recent focus on the gut microbiome, indole levels in fecal samples can also serve as a biomarker for indole-producing species in the gastrointestinal tract. One of the impediments in indole research has been the lack of a quantitative method that specifically detects indole that is simpler than the more expensive HPLC and mass spectrometry analysis currently used. In this report, we have demonstrated an easy, sensitive, and specific assay for measuring indole in complex biological samples. We anticipate that the HIA may also be used for indole detection in other biological samples, such as serum or urine (although not tested in this study).
The HIA did not detect the most common, naturally occurring indole analogs present in biological samples. In contrast, 1-methylindole, which is not found in bacterial cultures or human stool specimens, was detectable by HIA. We then used the HIA in proof-of-concept experiments to demonstrate its utility with bacterial cultures and with clinically relevant stool specimens. The mean and median fecal indole concentrations in 53 healthy adults were 2.59 mM and 2.73 mM, respectively. Fecal indole concentrations varied widely (0.30 mM to 6.64 mM) from person to person, perhaps indicating differences in the gut microbiota among humans. Few studies have reported indole concentrations in the stools of healthy adults. Gas chromatographic analysis of indole and 3-methylindole (skatole) from the fecal samples of 84 patients with colorectal cancers, 20 postsurgical cholecystectomy patients, and 15 healthy control patients reported concentrations in the 5- to 156-μg/g range for the two molecules (29, 30). These values are roughly consistent with what we found but cannot be compared directly since the data were expressed per gram (dry weight) of feces. By expressing data generated in the HIA in a standardized manner (i.e., millimolar), values from different laboratories and samples should be comparable.
The amount of indole produced by E. coli strain H10407 during a 24-h period was also determined using the HIA compared with the Kovács assay. From the same bacterial culture, the Kovács assay detected 4.7 ± 0.33 mM, compared to 3.3 ± 0.22 mM using the HIA. The discrepancy in the amounts of indole detected between the two methods is consistent with the known observation that the Kovács assay, although very sensitive, will also detect 3-methylindole (skatole), a common indole analog in biological materials, including bacterial cultures (18–25). These results suggest that 3-methylindole may be present in the culture medium and is probably responsible for increasing the estimation of indole by a significant percentage (30% in this experiment).
The major limitation of the HIA and the Kovács assay for indole quantitation is that the color of the reaction complex changes from pink to yellowish orange at high indole concentrations. This reduces the absorption maxima at 530 nm and makes quantitation inaccurate at higher concentrations. This limitation, however, can be overcome by diluting the sample and retesting at the lower indole concentration. In summary, the HIA should be useful for the accurate measurement of indole in a variety of biological materials, including the evaluation of various animal and human tissues and fluids.
ACKNOWLEDGMENTS
This work was supported by NIH R01 grant R01AI116914, discretionary funds from The University of Texas School of Public Health, the Texas Medical Center Digestive Diseases Center (Public Health Service grant DK56338), and the Gillson-Longenbaugh Foundation.
REFERENCES
- 1.Hirakawa H, Inazumi Y, Masaki T, Hirata T, Yamaguchi A. 2005. Indole induces the expression of multidrug exporter genes in Escherichia coli. Mol Microbiol 55:1113–1126. doi: 10.1111/j.1365-2958.2004.04449.x. [DOI] [PubMed] [Google Scholar]
- 2.Domka J, Lee J, Wood TK. 2006. YliH (BssR) and YceP (BssS) regulate Escherichia coli K-12 biofilm formation by influencing cell signaling. Appl Environ Microbiol 72:2449–2459. doi: 10.1128/AEM.72.4.2449-2459.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bansal T, Englert D, Lee J, Hegde M, Wood TK, Jayaraman A. 2007. Differential effects of epinephrine, norepinephrine, and indole on Escherichia coli O157:H7 chemotaxis, colonization, and gene expression. Infect Immun 75:4597–4607. doi: 10.1128/IAI.00630-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee J, Jayaraman A, Wood TK. 2007. Indole is an inter-species biofilm signal mediated by SdiA. BMC Microbiol 7:42. doi: 10.1186/1471-2180-7-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chant EL, Summers DK. 2007. Indole signalling contributes to the stable maintenance of Escherichia coli multicopy plasmids. Mol Microbiol 63:35–43. doi: 10.1111/j.1365-2958.2006.05481.x. [DOI] [PubMed] [Google Scholar]
- 6.Hirakawa H, Kodama T, Takumi-Kobayashi A, Honda T, Yamaguchi A. 2009. Secreted indole serves as a signal for expression of type III secretion system translocators in enterohaemorrhagic Escherichia coli O157:H7. Microbiology 155:541–550. doi: 10.1099/mic.0.020420-0. [DOI] [PubMed] [Google Scholar]
- 7.Vega NM, Allison KR, Khalil AS, Collins JJ. 2012. Signaling-mediated bacterial persister formation. Nat Chem Biol 8:431–433. doi: 10.1038/nchembio.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee JH, Lee J. 2010. Indole as an intercellular signal in microbial communities. FEMS Microbiol Rev 34:426–444. doi: 10.1111/j.1574-6976.2009.00204.x. [DOI] [PubMed] [Google Scholar]
- 9.Adijiang A, Higuchi Y, Nishijima F, Shimizu H, Niwa T. 2010. Indoxyl sulfate, a uremic toxin, promotes cell senescence in aorta of hypertensive rats. Biochem Biophys Res Commun 399:637–641. doi: 10.1016/j.bbrc.2010.07.130. [DOI] [PubMed] [Google Scholar]
- 10.Niwa T. 2010. Uremic toxicity of indoxyl sulfate. Nagoya J Med Sci 72:1–11. [PMC free article] [PubMed] [Google Scholar]
- 11.Wikoff WR, Anfora AT, Liu J, Schultz PG, Lesley SA, Peters EC, Siuzdak G. 2009. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc Natl Acad Sci U S A 106:3698–3703. doi: 10.1073/pnas.0812874106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller JM, Wright JW. 1982. Spot indole test: evaluation of four reagents. J Clin Microbiol 15:589–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reller LB, Mirrett S. 1975. Motility-indole-lysine medium for presumptive identification of enteric pathogens of Enterobacteriaceae. J Clin Microbiol 2:247–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.MacFaddin JF. 2000. Biochemical tests for identification of medical bacteria, 3rd ed. Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 15.Turner JM. 1961. A new reagent for the assay of indole in the tryptophanase reaction. Biochem J 78:790–792. doi: 10.1042/bj0780790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kleeberg J. 1982. Ehrlich's benzaldehyde reaction (with urobilinogen) 80 years later. Z Gastroenterol 20:424–428. (In German.) [PubMed] [Google Scholar]
- 17.Lamb AC, Federico-Perez RA, Xue ZL. 2015. Product in indole detection by Ehrlich's reagent. Anal Biochem 484:21–23. doi: 10.1016/j.ab.2015.04.033. [DOI] [PubMed] [Google Scholar]
- 18.Scott JE, Qian R, Henkel W, Glanville RW. 1983. An Ehrlich chromogen in collagen cross-links. Biochem J 209:263–264. doi: 10.1042/bj2090263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O'Neal CL, Crouch DJ, Fatah AA. 2000. Validation of twelve chemical spot tests for the detection of drugs of abuse. Forensic Sci Int 109:189–201. doi: 10.1016/S0379-0738(99)00235-2. [DOI] [PubMed] [Google Scholar]
- 20.Cross SN, Quinteros E, Roberts M. 2015. Surface modification for the collection and identification of fingerprints and colorimetric detection of urea nitrate. J Forensic Sci 60:193–196. doi: 10.1111/1556-4029.12558. [DOI] [PubMed] [Google Scholar]
- 21.Shah J, Jan MR, Khan I, Khan MN. 2012. Quantification of sparfloxacin in pharmaceutical dosages and biological samples. Pak J Pharm Sci 25:823–829. [PubMed] [Google Scholar]
- 22.Wu S, Sun J, Tong Z, Lan X, Shu B, Liu Y, Liao D. 2012. Rapid and simple colorimetric assay for screening angiotensin I-converting enzyme inhibitors. Pharm Biol 50:1303–1309. doi: 10.3109/13880209.2012.674534. [DOI] [PubMed] [Google Scholar]
- 23.Alqasaimeh M, Heng LY, Ahmad M, Raj AS, Ling TL. 2014. A large response range reflectometric urea biosensor made from silica-gel nanoparticles. Sensors (Basel) 14:13186–13209. doi: 10.3390/s140713186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fernandes FC, Silva AS, Rufino JL, Pezza HR, Pezza L. 2015. Screening and determination of sulphonamide residues in bovine milk samples using a flow injection system. Food Chem 166:309–315. doi: 10.1016/j.foodchem.2014.06.034. [DOI] [PubMed] [Google Scholar]
- 25.Hijarrubia MJ, Aparicio JF, Casqueiro J, Martin JF. 2001. Characterization of the lys2 gene of Acremonium chrysogenum encoding a functional alpha-aminoadipate activating and reducing enzyme. Mol Gen Genet 264:755–762. doi: 10.1007/s004380000364. [DOI] [PubMed] [Google Scholar]
- 26.DuPont HL, Chappell CL, Sterling CR, Okhuysen PC, Rose JB, Jakubowski W. 1995. The infectivity of Cryptosporidium parvum in healthy volunteers. N Engl J Med 332:855–859. doi: 10.1056/NEJM199503303321304. [DOI] [PubMed] [Google Scholar]
- 27.Okhuysen PC, Rich SM, Chappell CL, Grimes KA, Widmer G, Feng X, Tzipori S. 2002. Infectivity of a Cryptosporidium parvum isolate of cervine origin for healthy adults and interferon-gamma knockout mice. J Infect Dis 185:1320–1325. doi: 10.1086/340132. [DOI] [PubMed] [Google Scholar]
- 28.Okhuysen PC, Chappell CL, Crabb JH, Sterling CR, DuPont HL. 1999. Virulence of three distinct Cryptosporidium parvum isolates for healthy adults. J Infect Dis 180:1275–1281. doi: 10.1086/315033. [DOI] [PubMed] [Google Scholar]
- 29.Karlin DA, Mastromarino AJ, Jones RD, Stroehlein JR, Lorentz O. 1985. Fecal skatole and indole and breath methane and hydrogen in patients with large bowel polyps or cancer. J Cancer Res Clin Oncol 109:135–141. doi: 10.1007/BF00391888. [DOI] [PubMed] [Google Scholar]
- 30.Zuccato E, Venturi M, Di Leo G, Colombo L, Bertolo C, Doldi SB, Mussini E. 1993. Role of bile acids and metabolic activity of colonic bacteria in increased risk of colon cancer after cholecystectomy. Dig Dis Sci 38:514–519. doi: 10.1007/BF01316508. [DOI] [PubMed] [Google Scholar]