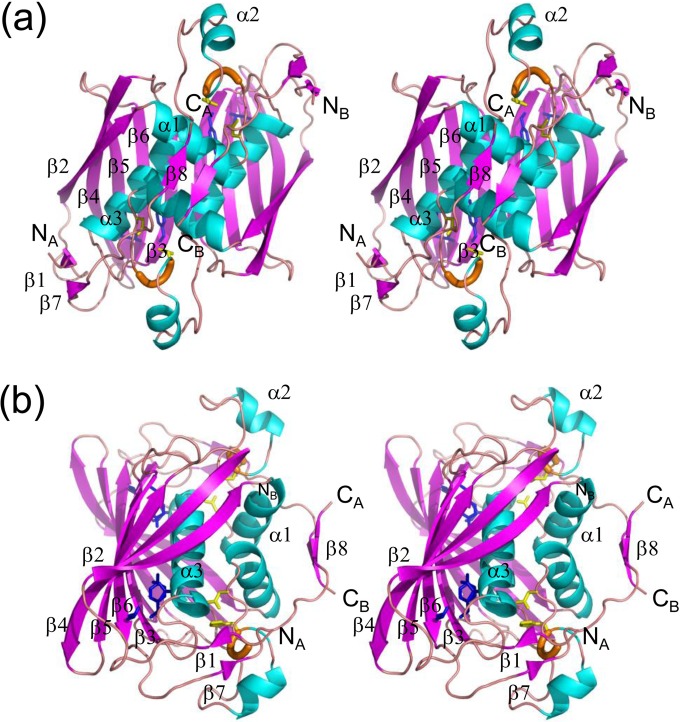
FIG 2.
Stereo diagrams of the crystal structure of the PhaJ1Pa dimer. α-Helices, β-strands, and 310-helices are indicated by helices (blue), arrows (magenta), and thick tubes (orange), respectively. The catalytic dyad residues Asp38 and His43 are represented by stick models (yellow). Residues located at the bottom of the acyl-chain-binding pocket (Ile72, Tyr83, and Ile136) also are represented by stick models (blue). N and C termini of each chain are labeled. Secondary structure annotations are shown only for chain A. (a) A front view of the structure along the 2-fold axis of symmetry. Two subunits adopt an almost identical conformation, with an RMSD of 0.38 Å for Cα atoms. (b) A side view of the structure, generated by 90° rotation relative to the image shown in panel a, highlighting the four-layered structure.