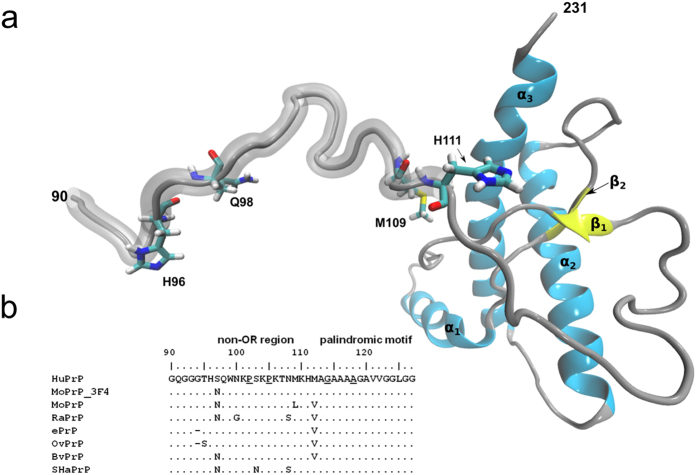
Figure 1. The non-OR region in the truncated HuPrP.
In (a) cartoon representation of the HuPrP(90–231) with highlighted the segment 90–111 including the apo form of the non-OR copper binding site. Residues H96, Q98, M109 and H111 contribute to coordinate one Cu(II) or Cu(I) ion. In (b), alignment of residues 90–127 including the non-OR region and the palindromic motif of different mammalian prion proteins (PrP): HuPrP (human, Homo sapiens, NCBI accession code AAA60182), MoPrP (mouse, Mus musculus, AAA39997) and MoPrP_3F4 (carrying the 3F4 tag), RaPrP (rabbit, Oryctolagus cuniculus, AAD01554.1), ePrP (elk, Cervus elaphus nelsoni, AAB94788), OvPrP (sheep, Ovis aries, AFM91142.1), BvPrP (bank vole, Myodes glareolus, AAL57231) and SHaPrP (Syrian hamster, Mesocricetus auratus, AAA37091). In the HuPrP primary sequence the residues involved in GSS-linked mutations are underlined (P102L, P105L, G114V and A117V).