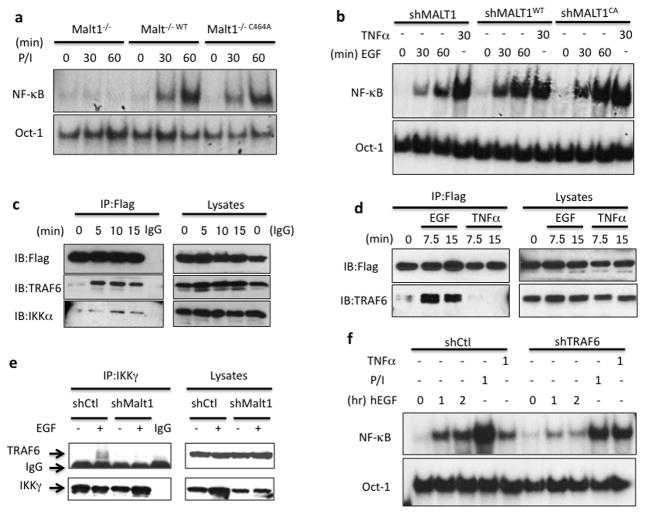
Figure 2. MALT1 serves as a scaffold protein by recruiting TRAF6 to IKK complex.
(a) MALT1-deficient cells (Malt1−/−), wild-type MALT1-reconstituted cells (Malt1−/−WT) and protease-deficient mutant reconstituted cells (Malt1−/−C464A) were stimulated with PMA and Ionomycin (50ng/ml; 100ng/ml) for indicated periods, respectively. Nuclear lysates were isolated and subjected to gel shift analysis for NF-κB activation. (b) A431 cells with a MALT1 knockdown (shMALT1), wild-type MALT1-reconstituted cells (shMALT1WT) and protease-deficient mutant reconstituted cells (shMALT1C464A) were stimulated with EGF (100ng/ml) or TNFα (10ng/ml) for indicated periods. Nuclear lysates were isolated and subjected to gel shift analysis for NF-κB activation. (c) MALT1-reconstituted cells were stimulated with EGF (100ng/ml) for indicated time and MALT1-Flag was immunoprecipitated (IP) by anti-Flag conjugated beads. The IP samples and lysates were analyzed by immunoblotting using the indicated antibodies. (d) MALT1-reconstituted cells were stimulated with EGF (100ng/ml) and TNFa, respectively. MALT1-Flag was immunoprecipitated (IP) by anti-Flag conjugated beads. The IP samples and lysates were analyzed by immunoblotting using the indicated antibodies. (e) Control (shCtl) or MALT1-silenced (shMALT1) A431 cells were either unstimulated or stimulated with EGF (100ng/ml) for 15 minutes and IKKg was immunoprecipitated (IP). The IP samples and lysates were analyzed by immunoblotting using the indicated antibodies. (f) A431 cells with a TRAF6 knockdown (shTRAF6) and control cells (shCtl) were stimulated with EGF (100ng/ml), PMA and Ionomycin (50ng/ml; 100ng/ml) or TNFα (10ng/ml) for indicated time points, respectively. NF-κB activation and Oct-1 (loading control) levels were determined by the gel shift assay.