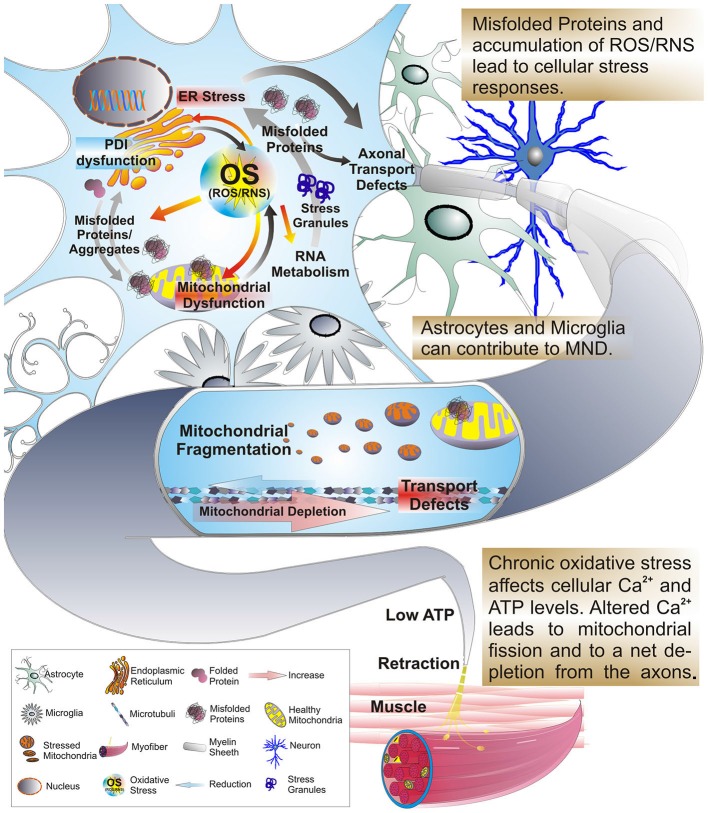
Figure 1.
Oxidative stress, protein misfolding and mitochondrial dysfunction are closely related. Excessive production of reactive oxygen or nitrogen species (ROS/RNS), transcriptional dysregulation, protein misfolding and ER stress can arise as consequences of OS and mitochondrial stress. In addition these factors work in a feedback-loop further exacerbating mitochondrial stress and dysfunction. A significant amount of mitochondrial proteins, including those of the ETC, contain highly oxidizable iron-sulfur-clusters that upon exposure to OS can be severely affected in their folding and function. But, OS also triggers stress responses in other organelles, such as the ER and persistent stress and highly oxidative conditions impair the function and integrity of protein folding in the ER. As a result the formation of misfolded proteins is favored leading to an accumulation of insoluble cytosolic and mitochondrial aggregates, impaired interference with activity of the PDI and impaired axonal transport. During the course of these alterations the levels of ATP and intra-cellular calcium are affected. This change interferes with the Ca2+ and ATP sensitive mitochondrial fusion/fission machinery and microtubule based mechanisms of mitochondrial transport. Mitochondria accumulate in the cell soma in a fragmented and dysfunctional state leading to a dramatic reduction of mitochondria transported anterograde to the axon terminal. Given the size of motor neurons with long axonal extensions, the impaired axonal transport leads to a depletion of functional mitochondria at the axon terminal. With the axonal periphery no longer supplied with sufficient ATP distal synapses degenerate eventually and the MN dies. As a consequence myofibers no longer receive input from their corresponding MN and are prone to atrophy, manifesting in increased muscle weakness of ALS patients.