Figure 3.
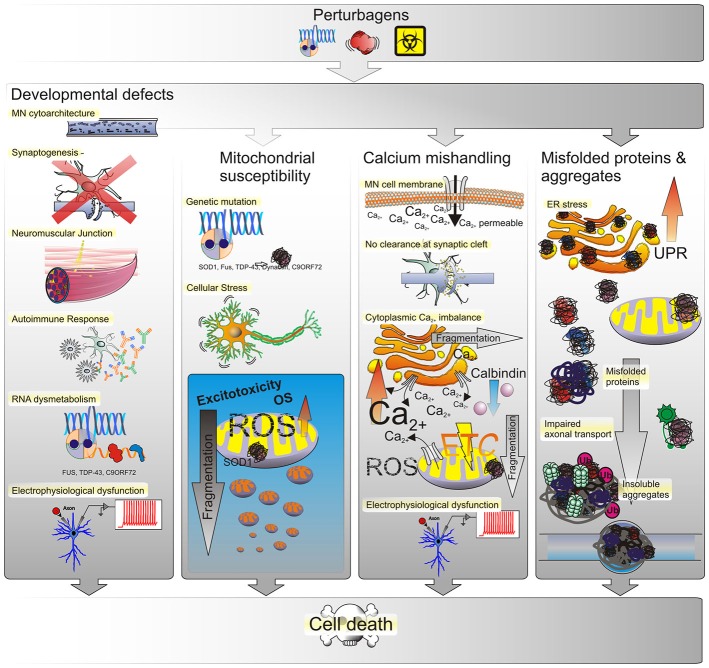
Possible causes of ALS. The most prevalent underlying reason for MN defects are genetic perturbagens, inherited or de novo mutations. Yet, the majority of ALS cases have not been linked to any mutation, suggesting that other effectors such as the environment may play into this as well. Thus MN phenotypes observed in ALS may arise from genetic and/or environmental perturbagens, as depicted in the top panel. Developmental anomalies may affect the structural integrity of neuronal cytoarchitecture as conferred by structural proteins, transport proteins, transmembrane proteins or by a disruption of RNA processing. Such defects can interfere or even prevent the formation of synapses between neurons or at the NMJ either directly within MN or indirectly by affecting neighboring glial cells. Triggering of autoimmune responses and chronic low-level inflammation may lead to MN degeneration as well and many of those developmental defects manifest in electrophysiological deficiencies such as a progressive decrease in voltage-activated Na+ and K+ currents correlated with a loss of functional outputs. Mitochondrial susceptibility due to ROS-induced OS in turn yields an inert vulnerability of MNs to excitotoxicity. An increased amount of mitochondrial stress in turn leads to mitochondrial fragmentation and ultimately cell death. Due to their large size and long neurite outgrowths, MNs are particularly sensitive to ion fluctuations, whether due to selective permeability for Ca2+ influx or lack of messenger clearance from the synaptic cleft. The dysregulation of intracellular Ca2+ levels has severe implications for MN function as well. Both, the ER and mitochondria function as buffers of cellular Ca2+ homeostasis. When intracellular Ca2+ levels increase, either by influx from the extracellular space or the ER and mitochondria, this triggers OS responses, their fragmentation and eventually progression of cell death signals that ultimately lead to loss of electrophysiological outputs. The generation of misfolded proteins and formation of aggregates, likewise affects the functional integrity of both organelles. Initially, misfolded proteins trigger the UPR in the ER to compensate for decreased protein translation and processing efficiency. Persistence of misfolded proteins that escape corrective degradation mechanisms cause accumulation in the cell and ultimately lead to the formation of insoluble aggregates interfering with cellular functions such as molecular transport and trafficking.