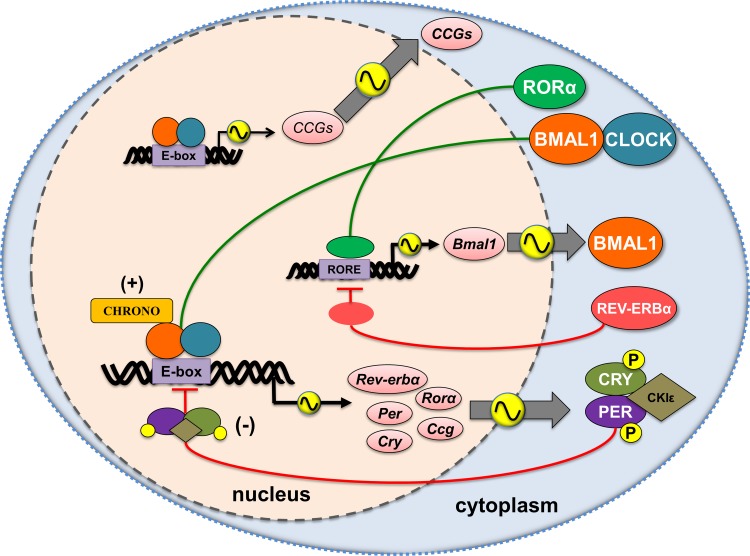
Fig. 1.
Schematic representation of the core clock mechanism and rhythmic output of key target genes that regulate the molecular clock function in the lung. The molecular clock in mammals is composed of a heterodimer of the transcription factors CLOCK and BMAL1, which regulates the transcription of Per (Period) and Cry (Cryptochrome) via E-box sequences within their promoters. PER and CRY heterodimerize and block E-box-dependent transcriptional activity of the CLOCK:BMAL1 complex. The timing of BMAL1 expression depends on the counteracting activity of REV-ERBs and ROR (retinoic acid receptor-related orphan receptor) proteins. CHRONO negatively regulates circadian oscillations by directly interacting with BMAL1. Environmental agents influence lung cells (e.g., epithelial cells) and macrophages through activation of kinases (MAP kinase, casein kinase2, CKII, glycogen synthase kinase GSK3 β, protein kinase C, and PI-3 kinase) that lead to posttranslational modification (phosphorylation/acetylation) of molecular clock proteins (e.g., BMAL1 and PER2). Molecular clock dysfunction negatively affects lung functions and may be a significant factor in the pathobiology of chronic airway diseases. CKIε/δ, casein kinase 1 ε/δ; CHRONO, ChIP-derived repressor of network oscillator; CLOCK, circadian locomotor output cycles kaput; CCG, clock controlled genes; CRY 1,2, cryptochrome 1,2; REV-ERBα, nuclear receptor subfamily 1, group D, member 1; PER1,2, period 1,2; RORα, retinoic acid receptor-related orphan receptor α.