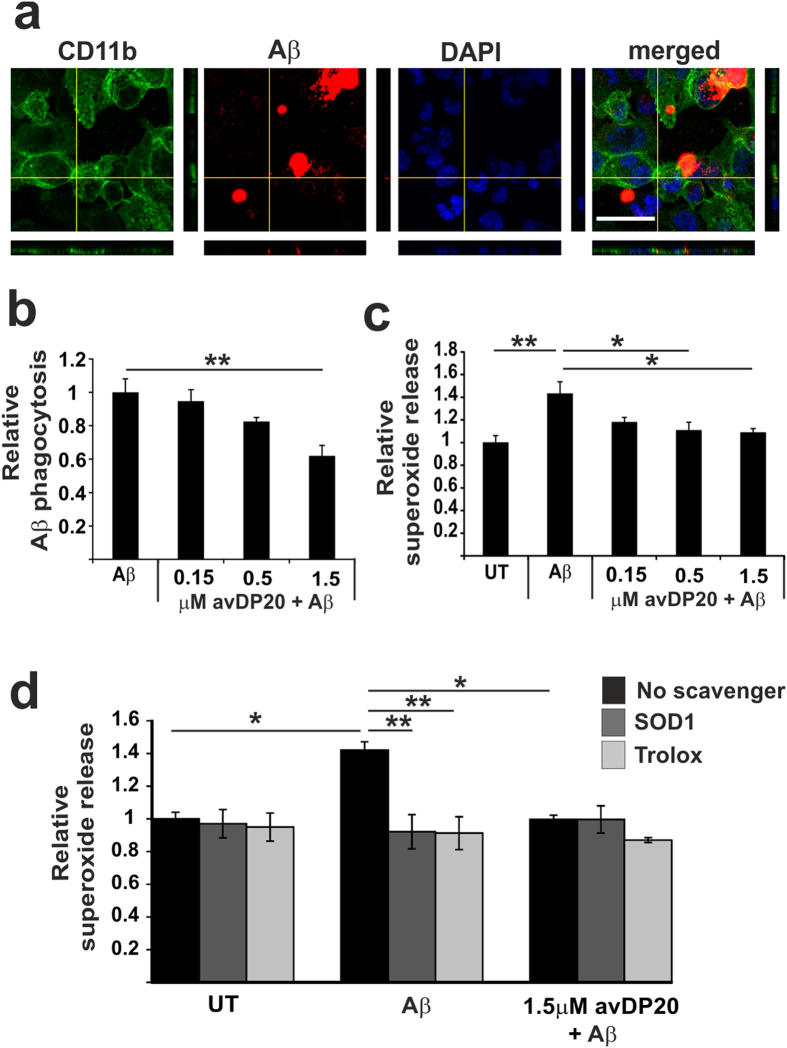
Figure 6. PolySia reduced macrophages phagocytosis of fibrillary amyloid-β1–42 and prevented the associated oxidative burst.
(a) Phagocytosis of fibrillary biotin-conjugated amyloid-β1–42 by human macrophages. Cells were fixed and stained with fluorescent labelled streptavidin and an antibody directed against CD11b. Nuclei were labelled by DAPI. Confocal 3D-reconstruction of a macrophage cell that showed ingested amyloid-β1–42 material inside the cell. Scale bar: 50 μm. (b) Quantification of the uptake of amyloid-β1–42 into macrophages. Cells were treated for 1 hour by polySia avDP20. PolySia avDP20 prevented the uptake of amyloid-β1–42 at a concentration of 1.5 μM. Data are presented as mean +/− SEM of n = 5 independent experiments. **p < 0.01, ANOVA followed by Bonferroni. (c) Release of superoxide as detected by DHE of the human macrophages challenged with fibrillary amyloid-β1–42 and treated with different concentrations of polySia avDP20. Superoxide release triggered by amyloid-β1–42 was inhibited by 0.5 μM and 1.5 μM polySia avDP20. Data are presented as mean +/− SEM of n = 6 independent experiments. * < 0.05, **p < 0.01, ANOVA followed by Bonferroni. (d) Scavenging of the superoxide release by superoxide dismutase SOD1 and Trolox. Human macrophages were challenged with fibrillary amyloid-β1–42 and superoxide release was determined by DHE. The radical scavenger Trolox and the SOD1 confirmed that DHE detected extracellular production of superoxide. Data are presented as mean +/− SEM of n = 3 independent experiments. * < 0.05, **p < 0.01; ANOVA followed by Bonferroni.