Abstract
Background:
Recent evidences have supported migraine headache and neurally mediated syncope as the especial types of endotheliopathies. To determine endothelial function in patients with migraine headache or those with neurally mediated syncope, the present study was conducted.
Materials and Methods:
This cross-sectional study was performed on 93 consecutive patients aged 5-20 years in four groups; neurally mediated syncope, migraine, both neurally mediated syncope and migraine, and control groups. All subjects were tested for basic biophysical and biochemical features including age, gender, body mass index, systolic, and diastolic blood pressures, intima-media thickness (IMT) and flow-mediated dilation (FMD), blood hemoglobin, fasting blood glucose, lipid profile, intercellular adhesion molecule (ICAM), vascular cell adhesion molecule (VCAM), and E-selectin.
Results:
The mean levels of VCAM and ICAM were significantly higher in all groups when compared to control group (P < 0.05). FMD was significantly higher in syncope, migraine, and syncope and migraine groups than in the control group (P < 0.05). Furthermore, mean IMT was significantly lower in migraine and also in syncope and migraine groups than in syncope group and control group (P < 0.05). Examining the association between IMT and other baseline parameters showed positive association of IMT with systolic and diastolic blood pressures.
Conclusion:
Endothelial dysfunction is seen in both migraine headache and neurally mediated syncope. Changes in endothelial functional indices are also dependent on the blood pressure.
Keywords: Endothelial function, flow-mediated dilation, intima-media thickness, migraine headache, syncope
INTRODUCTION
Migraine headache is a common disorder with neurovascular fundament, manifested by repeated episodes of moderate to severe headache as well as autonomic nervous system dysfunction.[1] Although the basic pathophysiology of this disease remained unclear, but recent evidences have emphasized its vascular sources, especially vasospastic stimulation, and endothelial dysfunction.[2] In this regard, it has been named and subcategorized as endotheliopathy.[3] It is now suggested that in response to stimuli or endothelial damage, some specific vasoactive mediators such as endothelin-1 and nitric oxide may be secreted from endothelial cells that are responsible for appearing or deteriorating the migraine headache.[4,5,6] The assessment of raising of these mediators has been recommended to predict early endothelial dysfunction in those with an initial migraine attack. Besides, in describing pathophysiological basis of syncope, it has been hypothesized the modulation of endothelial function can be accompanied with an impairment of vascular tonicity of peripheral arteries leading to improper peripheral vasomotion which identified as the main pathogenesis of syncope.[7] Similar to the suggested basis for a migraine headache, an abnormal peripheral vasoreactivity has been shown to be associated with increased risk for syncope particularly in its neurally mediated type.[8]
In 2009, Yaghini et al.[9] studied 4096 students aged 11-18 years old in Isfahan, Iran. Of 2047, 49.9% had headaches which the prevalence of migraine headache was 19.3%, and the frequency of migraine headache was significantly higher among girls as compared to boys. The author experience is that the chance of migraine headache is more in neurally mediated syncope patients than the general population. To clearly determine endothelial function in patients with recurrent migraine headache or those with neurally mediated syncope or both, the present cross-sectional study was conducted. In fact, by demonstrating the central role of endothelial functional indicators, which predispose the patients to migraine or neutrally mediated syncope, it will be feasible to provide new opportunities for the treatment of these diseases.
MATERIALS AND METHODS
Study population
This cross-sectional study was performed on 93 consecutive children and young adults aged 5-20 years in four different groups. In the first group 22 subjects with neurally mediated syncope (with positive tilt test), in second group 21 subjects with migraine headache, in third group 20 subjects with both neurally mediated syncope (with positive tilt test) and migraine, and in fourth group 30 sex- and age-matched healthy subjects as controls were involved which referred to one of the Shahid Chamran and Imam Hossein Hospital in Isfahan in 2014.
The first group (subjects with neurally mediated syncope) was tilt test positive and referred from a pediatric cardiologist.
The second group (migraine group) cases were selected consecutively from children and young adults aged 5-20 years referred to the Imam Hossein Hospital and diagnosed as migraine headache according to the second edition of the International Classification of Headache Disorders.[1] The subjects in the third group (both neurally mediated syncope and migraine) had all criteria of both migraine headache and neurally mediated syncope. Furthermore, sex- and age-matched healthy groups without any evidences of the migraine and neurally mediated syncope disease conditions.
Study measurements
The study was approved by Local Ethics Committee of Isfahan University of Medical Sciences (research project number: 393525). After explaining the objectives of the study and obtaining written informed consent from patients or their parents and guardians, baseline characteristics, and clinical data of participants were collected by interviewing and recorded at the study questionnaire. The patients also underwent supplementary diagnostic tests including electrocardiography (ECG) for ruling out arrhythmias and echocardiography for ruling out structural heart diseases. Furthermore, the patients neurologically examined by a pediatric neurologist. Patients with a history of true seizure, abnormal physical exams, abnormal ECG, electroencephalogram, echocardiography, psychological problems, and those who had negative tilt test or had any associated diseases, which had influences on the study goals were omitted. Other parameters were measured by biophysical and biochemical tests. For biophysical parameters, blood pressure was measured three times on both arms after 20 min of rest. Seca scale was used to measure the weight, height was measured with a tape measure, and body mass index (BMI) was calculated as weight/height² (kg/m²).
The assessment of endothelial functional state was done by ultrasound system (Medison EKO 7) equipped with vascular software for two-dimensional (2D) imaging and a linear array transducer with a frequency of 7 MHz. A blood pressure cuff was first placed on the forearm. A baseline rest image of brachial artery's lumen diameter was acquired, and blood flow was estimated by the pulsed Doppler velocity signal obtained from a mid-artery sample volume. Thereafter, the cuff was inflated to at least 50 mmHg above systolic pressure to occlude arterial inflow for 5 min. Subsequently, the cuff was deflated. A mid-artery pulsed Doppler signals was obtained after 60 s the cuff release to assess hyperemic velocity. Percentage increase in lumen diameter during postischemic hyperemia as compared to basal lumen diameter was labeled as flow-mediated dilation (FMD). FMD percentage is calculated using the following formula:
FMD% = (h max − b)/b × 100
Where, h max equals the maximum diameter of the vessel, and b equals the measured baseline diameter.[10]
To assess the intima-media thickness (IMT), on a longitudinal, 2D ultrasound image of the carotid artery, the anterior (near,) and posterior (far) walls of the carotid artery are displayed as two bright white lines separated by a hypoechogenic space. The distance between the leading edge of the first bright line of the far wall (lumen-intima interface) and the leading edge of the second bright line (media-adventitia interface) indicates the IMT. We measured the IMT of the right common carotid artery just before the bifurcation three times and the average was taken as the value of IMT for our study.
For biochemical parameters, a 20 ml blood sample was taken after the fasting state for measuring serum levels of E-selectin, vascular cell adhesion molecule (VCAM), and intercellular adhesion molecule (ICAM) by enzyme-linked immunosorbent assay (Boster Immunoleader, Pleasanton, CA, USA) according to manufacturer protocols. Hemoglobin was measured by hematology analyzer. Fasting blood sugar was measured by glucose oxidase method and lipid profile including triglyceride, total cholesterol, and high-density lipoprotein (HDL)- and low-density lipoprotein (LDL)-cholesterol was measured by enzymatic method (Pars Azmoon, Tehran, Iran) according to manufacturer protocols using automated analyzer.
Statistical analysis
Using statistical analysis software SPSS (version 20, SPSS Inc., Chicago, IL), descriptive statistics and frequencies are performed for continuous and discrete variables, respectively. Normality of variables was tested by Kolmogorov-Smirnov and Shapiro-Wilk test. Comparing the mean values between independent groups was analyzed statistically by Student's t-test. Pearson correlation coefficient test was used for the detection of association between variables in each group. A P < 5% was taken as significant.
RESULTS
A total of 93 individuals were included into the study and categorized on the final diagnosis in one of the following four groups:
Patients with syncope alone (n = 22),
Patients with migraine headache alone (n = 21),
Patients with both migraine headache and syncope simultaneously (n = 20), and
Healthy ones as the controls (n = 30).
Basic characteristics
In the first group, family history of syncope was revealed in 18.2%. The majority of the patients had pallor (77.3%) during the attack, while abdominal pain, sweating, and nausea were observed in 9.1%, 9.1%, and 4.5% of them, respectively. Mean duration of syncope manifestations was 20.55 months ranged widely from 14 days to 7 years before they were referred to the pediatric cardiologist.
In the second group, 33.3% experienced 1 episode of migraine headache per week, 33.3% experienced 2 episodes per week, 14.3% experienced 3 episodes per week, and 19.1% had more episodes of headache per week. Regarding the type of a migraine headache, headache was pulsatory in 42.9% and nonpulsatory in 57.1%. Furthermore, nausea alone, vomiting alone, and both symptoms were observed in 14.3%, 23.8%, and 61.9%, respectively.
In the third group, family history of syncope was revealed in 15%. Like the first group, the majority of the patients had pallor (80%) during the syncope episodes, although nausea and vertigo were observed in 45% and 5% of them, respectively. Mean duration of syncope manifestations was 31.44 months ranged widely from 7 days to 10 years before they were referred to the pediatric cardiologist. During the migraine attacks, 25% experienced 1 episode per week, 10% experienced 2 episodes per week, and 20% experienced 3 episodes per week. Regarding the type of migraine headache, headache was pulsatory in 20% and nonpulsatory in 80%. Control subjects were asymptomatic.
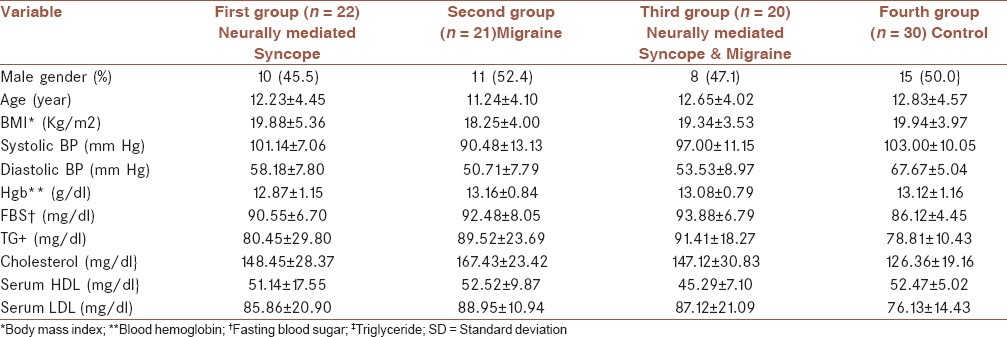
All four study groups with syncope alone, migraine headache alone, both migraine headache and syncope simultaneously, and the healthy group were similar in gender and age distribution. Furthermore, there was no difference across the groups in terms of BMI [Table 1].
Table 1.
All variables reported as mean ± SD
Biochemical characteristics
The results of biochemical endothelial function parameters between the study groups are summarized in Figures 1–3. In this regard, mean VCAM was significantly higher in syncope, migraine, migraine and syncope groups when compared with control group (P = 0.005, 0.038, and 0.031, respectively). Furthermore, mean levels of ICAM in syncope, migraine, and syncope and migraine groups were significantly higher when compared with control group (P = 0.02, 0.016, and 0.028, respectively). There were no significant differences among groups regarding to E-selectin. Difference across the groups in terms of serum hemoglobin level, fasting blood glucose, serum triglyceride, and also serum HDL and LDL levels did not seen (P > 0.05). Those patients with syncope, migraine, and syncope and migraine had significantly higher serum cholesterol level than the control group (P = 0.039, 0.026, and 0.042, respectively).
Figure 1.

Serum vascular cell adhesion molecule ng/ml in syncope, migraine, syncope and migraine, and control groups. Standard error of the mean is shown as the error bar. Data are shown as means ± standard deviation. *P < 0.05 versus control
Figure 3.

Serum E-selectin ng/ml in syncope, migraine, syncope and migraine, and control groups. Standard error of the mean is shown as the error bar. Data are shown as means ± standard deviation
Figure 2.
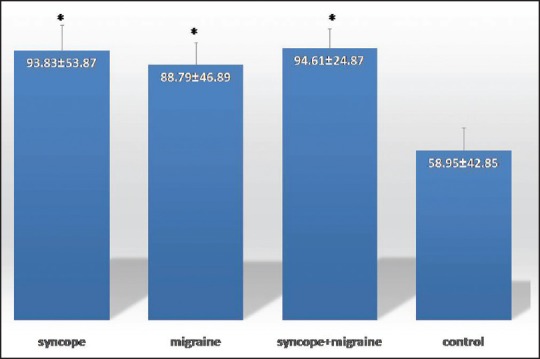
Serum intercellular adhesion molecule ng/ml in syncope, migraine, syncope and migraine, and control groups. Standard error of the mean is shown as the error bar. Data are shown as means ± standard deviation. *P < 0.05 versus control
Biophysical characteristics
Mean FMD was significantly higher in syncope, migraine headache, and syncope and migraine headache groups when compared with control group (P < 0.001, <0.001, and =0.003, respectively) [Figure 4]. Furthermore, assessing the correlation between FMD and other parameters showed that FMD was positively associated with serum cholesterol level and was adversely associated with diastolic blood pressure [Table 3]. Examining the association between IMT and other baseline parameters [Table 2] showed positive association of IMT with systolic and diastolic blood pressures. Furthermore, mean IMT [Figure 5] was significantly higher in syncope (P = 0.006) and control (P < 0.001) groups in comparison to migraine headache and, syncope, and migraine headache groups but there was no significant differences between IMTs in syncope alone and control group (P > 0.05). Those patients with migraine headache had significantly lower systolic and diastolic blood pressure than other study groups (P < 0.001).
Figure 4.

Flow-mediated dilatation in syncope, migraine, syncope and migraine, and control groups. *P ≤ 0.05 versus control, **P ≤ 0.001 versus control. Standard error of the mean is shown as the error bar. Data are shown as means ± standard deviation
Table 3.
Pearson rank order correlation coefficients and P value among flow-mediated diameter and baseline characteristics
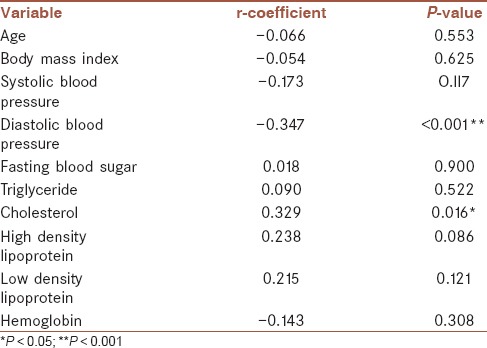
Table 2.
Pearson rank order correlation coefficients and P value among intima-media thickness and baseline characteristics
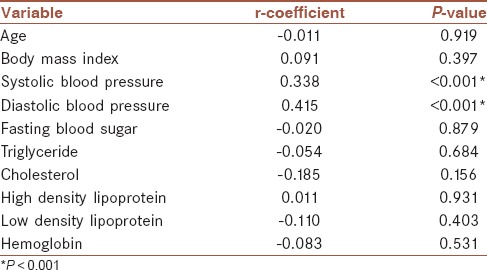
Figure 5.
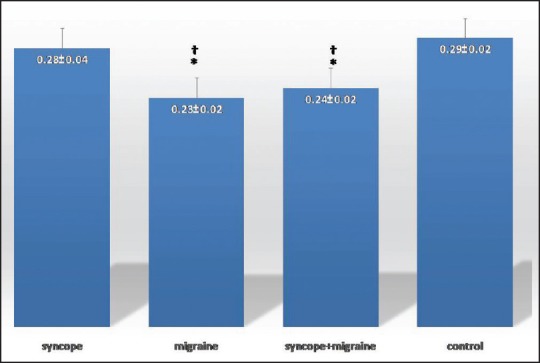
Intima-media thickness in syncope, migraine, syncope and migraine, and control groups. *P ≤ 0.05 versus syncope †P ≤ 0.001 versus control. Standard error of the mean is shown as the error bar. Data are shown as means ± standard deviation
DISCUSSION
In this, some strong evidences were obtained about endothelial dysfunction in both groups of patients with migraine headache and those with neurally mediated syncope. These documented findings were achieved by assessing endothelial function parameters including IMT, FMD, ICAM, and VCAM measurement. Furthermore, impairment of endothelial function in migraine headache patients was predicted by significantly elevated ICAM and VCAM, higher FMD, and lower IMT. Endothelial impairment in syncope patients was envisioned by elevating ICAM, VCAM, and FMD parameters.
Regarding susceptibility of migraine headache patients to endothelial dysfunction, the main fundamental changes in endothelial cells have been shown to be gradual reduction in the number and function of endothelial progenitor cells, serving as a marker for dysfunction of the endothelium.[11] Furthermore, endothelial dysfunction in these patients might be related to arterial hypersensitivity to nitric oxide following autonomic dysfunction leading to impairment of sympathetic control of blood flow.[12,13,14] However, these documents have not been revealed in some studies and may be due to the difference in intensity and episodes of migraine attacks.[15] Although our study introduces lowering IMT index in a migraine headache, but in some studies, an increase of this parameter in adults was shown[16,17] and in some others, it was unchanged.[18] As shown in the present study, because these parameters are considerably affected by the systolic and diastolic blood pressure, the association between endothelial function and these parameters may be potentially confounded by blood pressure changes.
Regarding association between endothelial dysfunction and its main determinants in patients with neutrally mediated syncope, although the relationship of FMD and ICAM, VCAM changes with endothelial dysfunction was demonstrated in our study, but the results of previous studies were contradictory. Similarly, Takase et al.[7] showed that FMD in adult patients with neurally mediated syncope was significantly greater than those in controls. Furthermore, Galetta et al.[19] indicated higher FMD in neurally mediated syncope group than in control group. In Santini et al. study,[8] the evaluation of endothelial function supports evidence that neuroleptic malignant syndrome is characterized by marked and sustained endothelial-independent vasodilation, in the presence of a normal FMD. All of these studies were done in adult. Our unpublished study in children also showed significantly higher amounts of FMD and biochemical markers in syncope patients in comparison with normal healthy control group.[20]
According to our knowledge, there is no published study that evaluates these biochemical markers in syncope and/or migraine in pediatric patients.
CONCLUSION
Endothelial dysfunction is present in both migraine headache and syncope. The therapeutic measures which modulate the endothelial function might be effective to control the attacks in these problems. There are nonpharmacological options to treat or reduce the syncopal episodes in syncope, and this measure might be effective for the prevention of migraine headache.
STUDY LIMITATIONS
Changes in endothelial functional indices may be affected by changes in blood pressure or elevated cholesterol level. Because of our partially small sample size, these claims were not able to test by employing multivariate regression models that should be considered in further studies. Furthermore, we did not follow our patient's during the treatment to know if the treatments have any influence on the endothelial function changes.
Financial support and sponsorship
This research was supported by grant 393525 from Isfahan University of Medical Sciences to Dr. Mohamad Reza Sabri.
Conflicts of interest
There are no conflicts of interest.
AUTHOR'S CONTRIBUTION
All authors had a contribution in all stages of the work. All of them contributing in preparing the draft of the manuscript and confirmed it.
Acknowledgments
We sincerely, appreciate Hajar Naji and Mohamadhasan Tajadini.
REFERENCES
- 1.Silberstein SD. Migraine. Lancet. 2004;363:381–91. doi: 10.1016/S0140-6736(04)15440-8. [DOI] [PubMed] [Google Scholar]
- 2.Pietrobon D, Striessnig J. Neurobiology of migraine. Nat Rev Neurosci. 2003;4:386–98. doi: 10.1038/nrn1102. [DOI] [PubMed] [Google Scholar]
- 3.Landmesser U, Hornig B, Drexler H. Endothelial function: A critical determinant in atherosclerosis? Circulation. 2004;109(21 Suppl 1):II27–33. doi: 10.1161/01.CIR.0000129501.88485.1f. [DOI] [PubMed] [Google Scholar]
- 4.Gabrielli M, Santarelli L, Addolorato G, Foschi G, Di Gampli C, Gasbarrini A, et al. High prevalence of antiendothelial cell antibodies in migraine. Headache. 2002;42:385–6. doi: 10.1046/j.1526-4610.2002.02114.x. [DOI] [PubMed] [Google Scholar]
- 5.Gallai V, Sarchielli P, Firenze C, Trequattrini A, Paciaroni M, Usai F, et al. Endothelin 1 in migraine and tension-type headache. Acta Neurol Scand. 1994;89:47–55. doi: 10.1111/j.1600-0404.1994.tb01632.x. [DOI] [PubMed] [Google Scholar]
- 6.Tietjen GE, Al-Qasmi MM, Athanas K, Dafer RM, Khuder SA. Increased von willebrand factor in migraine. Neurology. 2001;57:334–6. doi: 10.1212/wnl.57.2.334. [DOI] [PubMed] [Google Scholar]
- 7.Takase B, Akima T, Uehata A, Katushika S, Isojima K, Satomura K, et al. Endothelial function and peripheral vasomotion in the brachial artery in neurally mediated syncope. Clin Cardiol. 2000;23:820–4. doi: 10.1002/clc.4960231131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Santini L, Capria A, Brusca V, Violo A, Smurra F, Scarfò I, et al. An increased endothelial-independent vasodilation is the hallmark of the neurally mediated syncope. Clin Cardiol. 2012;35:107–10. doi: 10.1002/clc.20990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yaghini O, Shahkarami SM, Mahmoudian T, Hashemi EH. Comprehensive assessment of the relative frequency of sleep disorders in migraine and non migraine in 6-to 14-year-old children. Iran J Child Neurol. 2011;4:33–6. [Google Scholar]
- 10.Karvonen T, Matias T, Seppänen T, Tiinanen S, Kiviniemi A, Okamoto K, et al. Ultrasound image analysis for assessment of flow-mediated dilation of human arteries. 2013;51(Supplement):R-249. [Google Scholar]
- 11.Lee ST, Chu K, Jung KH, Kim DH, Kim EH, Choe VN, et al. Decreased number and function of endothelial progenitor cells in patients with migraine. Neurology. 2008;70:1510–7. doi: 10.1212/01.wnl.0000294329.93565.94. [DOI] [PubMed] [Google Scholar]
- 12.Rodríguez-Osorio X, Sobrino T, Brea D, Martínez F, Castillo J, Leira R. Endothelial progenitor cells: A new key for endothelial dysfunction in migraine. Neurology. 2012;79:474–9. doi: 10.1212/WNL.0b013e31826170ce. [DOI] [PubMed] [Google Scholar]
- 13.Thomsen LL, Iversen HK, Brinck TA, Olesen J. Arterial supersensitivity to nitric oxide (nitroglycerin) in migraine sufferers. Cephalalgia. 1993;13:395–9. doi: 10.1046/j.1468-2982.1993.1306395.x. [DOI] [PubMed] [Google Scholar]
- 14.Liman TG, Neeb L, Rosinski J, Wellwood I, Reuter U, Doehner W, et al. Peripheral endothelial function and arterial stiffness in women with migraine with aura: A case-control study. Cephalalgia. 2012;32:459–66. doi: 10.1177/0333102412444014. [DOI] [PubMed] [Google Scholar]
- 15.Besir FH, Koçer A, Dikici S, Yazgan S, Ozdem S. The evaluation of atherosclerosis in migraine patients. Pain Pract. 2013;13:41–5. doi: 10.1111/j.1533-2500.2012.00551.x. [DOI] [PubMed] [Google Scholar]
- 16.Hamed SA, Hamed EA, Ezz Eldin AM, Mahmoud NM. Vascular risk factors, endothelial function, and carotid thickness in patients with migraine: Relationship to atherosclerosis. J Stroke Cerebrovasc Dis. 2010;19:92–103. doi: 10.1016/j.jstrokecerebrovasdis.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 17.Malik AR, Kondragunta V, Kullo IJ. Forearm vascular reactivity and arterial stiffness in asymptomatic adults from the community. Hypertension. 2008;51:1512–8. doi: 10.1161/HYPERTENSIONAHA.107.106088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiménez Caballero PE, Muñoz Escudero F. Peripheral endothelial function and arterial stiffness in patients with chronic migraine: A case-control study. J Headache Pain. 2013;14:8. doi: 10.1186/1129-2377-14-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galetta F, Franzoni F, Plantinga Y, Ghiadoni L, Merico G, Tocchini L, et al. Endothelial function in young subjects with vaso-vagal syncope. Biomed Pharmacother. 2006;60:448–52. doi: 10.1016/j.biopha.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 20.Dehghan B, Sabri MR, Javanmard SH, Ahmadi AR, Mansourian M. Neurally mediated syncope. “Is it really an endothelial dysfunction?”. 2015 doi: 10.5152/AnatolJCardiol.2015.6325. [DOI] [PMC free article] [PubMed] [Google Scholar]


