Fig. 2.
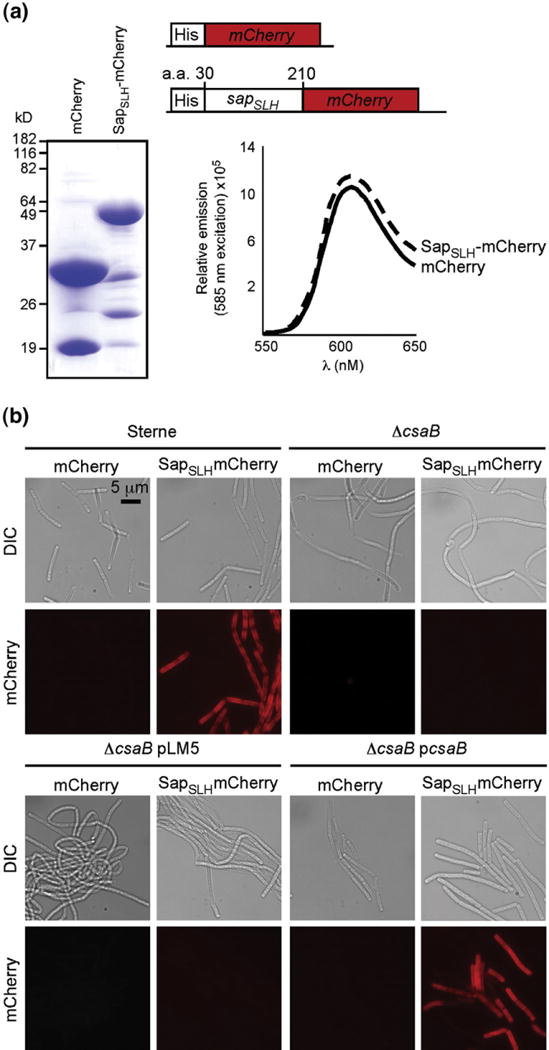
Purified, recombinant SapSLH–mCherry, but not mCherry, binds to the cell walls of B. anthracis strains harboring csaB. (a) Coomassie-stained SDS-PAGE of hexahistidyl-purified mCherry and a translational hybrid between the Sap SLH domains (amino acids 30 to 210 of the sap gene) and mCherry. Abundant, soluble forms of both proteins were purified from E. coli and, at equal concentrations, exhibited equivalent fluorescent yields with identical peak excitation (585 nm) and emission (608 nm) spectra. (b) Fluorescent micrographs of B. anthracis Sterne, ΔcsaB, ΔcsaB pLM5, and ΔcsaB pcsaB cells incubated with 1 μM mCherry or SapSLH–mCherry. Staining of the cell wall depends upon the presence of the SLH domain and the csaB gene, validating the use of this as an in vitro model of S-layer assembly.
