Figure 6.
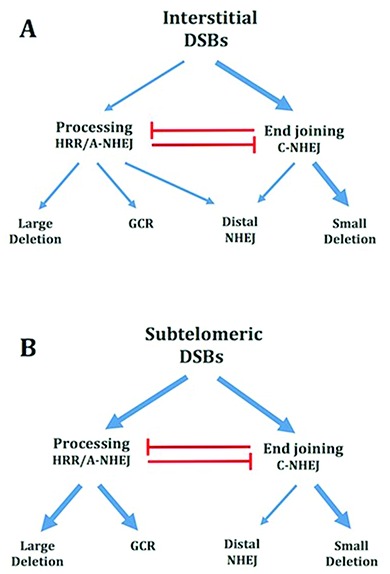
Model for the mechanisms of formation of mutations during repair of interstitial and subtelomeric I-SceI-induced DSBs. (A) Mechanisms of formation of mutations at interstitial DSBs. DSB repair occurs either directly through C-NHEJ, or following the processing of the ends of the DSB. As with HRR, large deletions and GCRs also involve the processing of DSBs, however repair occurs by A-NHEJ. Importantly, the GCR assay does not detect GCRs that also involve large deletions. However, large deletions near telomeres also commonly result in GCRs, so that the major difference between the large deletion and GCR assays is the extent of degradation involved in the GCR. Small deletions of a few base pairs occur during end joining involving C-NHEJ. Distal NHEJ (deletions resulting from joining two closely positioned DSBs) occurs both by end joining by C-NHEJ and following processing and A-NHEJ. (B) Mechanisms of formation of mutations at subtelomeric DSBs. End joining by C-NHEJ at subtelomeric DSBs occurs with the same efficiency as at interstitial DSBs, as shown by the fact that small deletions at subtelomeric DSBs occur at the same frequency as at interstitial DSBs. The repair of subtelomeric DSBs by end joining by C-NHEJ is ATM-dependent. As at interstitial DSBs, large deletions and GCRs occur through processing of DSBs and A-NHEJ, although with a much greater frequency than at interstitial DSBs. The decreased frequency of distal NHEJ at subtelomeric DSBs appears to be due to a reduced contribution of A-NHEJ, possibly because most DSBs repaired by A-NHEJ at subtelomeric DSBs become large deletions and/or GCRs. Combined together, our results suggest that the sensitivity of subtelomeric regions to DSBs is a result of excessive processing by MRE11 and other nucleases, and is not due to a deficiency in C-NHEJ.
