Abstract
Objectives
To determine the medical costs of laboratory-confirmed rotavirus hospitalizations and emergency department (ED) visits and estimate the economic impact of the rotavirus vaccine program.
Patients and methods
During 4 rotavirus seasons (2006–2009), children <3 years of age hospitalized or seen in the ED with laboratory-confirmed rotavirus were identified through active population-based rotavirus surveillance in three US counties. Medical costs were obtained from hospital and physician billing data, and factors associated with increased costs were examined. Annual national costs were estimated using rotavirus hospitalization and ED visit rates and medical costs for rotavirus hospitalizations and ED visits from our surveillance program for pre- (2006–2007) and post-vaccine (2008–2009) time periods.
Results
Pre-vaccine, for hospitalizations, the median medical cost per child was $3581, the rotavirus hospitalization rate was 22.1/10,000, with an estimated annual national cost of $91 million. Post-vaccine, the median medical cost was $4304, the hospitalization rate was 6.3/10,000 and the estimated annual national cost was $31 million. Increased costs were associated with study site, age <3 months, underlying medical conditions and an atypical acute gastroenteritis presentation. For ED visits, the pre-vaccine median medical cost per child was $574, the ED visit rate was 291/10,000 resulting in an estimated annual national cost of $192 million. Post-vaccine, the median medical cost was $794, the ED visit rate was 71/10,000 with an estimated annual national cost of $65 million.
Conclusions
After implementation of rotavirus immunization, the total annual medical costs decreased from $283 million to $96 million, an annual reduction of $187 million
Keywords: Rotavirus, Medical costs, Hospitalizations, Emergency department visits, NVSN
1. Introduction
Prior to implementation of the rotavirus immunization program in United States (US), rotavirus was the most common cause of severe gastroenteritis in young children resulting in 55,000–70,000 hospitalizations, and 205,000–272,000 emergency department (ED) visits each year with estimated medical costs in children less than five years of age of $264–319 million dollars [1–3]. The recent licensure and universal use of two rotavirus vaccines heighten the importance of examining the impact of the US rotavirus immunization program on health care costs [4].
In the past, rotavirus-associated hospitalization and ED costs have been estimated from administrative databases using ICD-9 codes for diarrhea, then using one of two indirect methods to estimate the number of rotavirus diarrhea cases [5–14]. The winter residual method estimates the number of rotavirus cases by comparing the number of hospitalizations for diarrhea during the year with the excess during the months when rotavirus gastroenteritis is prevalent [5]. The proportional method uses monthly proportions of rotavirus-positive stool samples from a large surveillance study [15] and applies these to the number of diarrhea-related hospitalizations. These indirect estimates may not be accurate for several reasons. With improvements in diagnostic testing, the proportion of rotavirus-positive children may be higher than reported in the past. If the rotavirus season is indistinct, the winter residual method would underestimate cases. Imperfect sensitivity or specificity with diarrhea codes may lead to inaccurate estimates of diarrhea cases. A rotavirus-specific ICD-9 code for rotavirus diarrhea was introduced in 1992 [7]. Studies have utilized this code as a direct method to estimate rotavirus disease [7–15] and compared to rotavirus active surveillance this code was specific, but greatly underestimated rotavirus disease in both hospital and outpatient settings [16,17]. To overcome these limitations, active surveillance studies determined the proportion of acute gastroenteritis cases due to laboratory-confirmed rotavirus [17–22] and estimated rates of rotavirus-associated hospitalizations and ED visits [19–22]. In both settings, these studies have shown the proportion of rotavirus-positive cases to be much higher than earlier studies. Since past cost data have also used data from these administrative datasets, the accuracy of these cost estimates is unclear.
Since 2006, the New Vaccine Surveillance Network (NVSN) has conducted prospective population-based active surveillance in three US counties to identify children with laboratory-confirmed rotavirus infection [20–22]. This system overcomes the limitations of previous studies by examining the economic burden of hospitalizations and ED visits associated with laboratory-confirmed population-based rotavirus disease rates in young children, identifying factors leading to the most costly hospitalizations, and estimating the impact of the rotavirus immunization program on health care costs.
2. Methods
2.1. Study design and population
The design and methods of the NVSN active population-based surveillance program have been previously described [20–22]. Briefly, population-based surveillance of children <3 years of age with vomiting or diarrhea was conducted at participating NVSN sites: Cincinnati Children's Hospital Medical Center (Cincinnati), Vanderbilt University Medical Center (Nashville), and the University of Rochester Medical Center (Rochester). Surveillance was conducted over four seasons: 2006 (December 2005–June 2006), 2007 (December 2006–June 2007), 2008 (December 2007–June 2008), and 2009 (July 2008–June 2009). Participating NVSN hospitals captured >95% of all pediatric hospital admissions in their respective counties, but varied in the proportion of pediatric ED visits captured (Hamilton County, Ohio, 95% at Cincinnati Children's Hospital Medical Center ED; Davidson County, Tennessee, 54% at Vanderbilt University Medical Center ED; Monroe County, New York, 70% at Strong Memorial ED and 25% at Rochester General ED). Inpatient children were enrolled five days a week while ED children were systematically enrolled every second to fourth day.
Children eligible for enrollment for acute gastroenteritis surveillance were <3 years of age, residents of the specified counties and presented with symptoms of acute gastroenteritis. Children were excluded if they were immunocompromised, hospitalized in the prior 4 days or transferred from another hospital after 48 h of admission, were never discharged from the hospital, had symptoms for >10 days, or had a non-infectious or other identifiable cause of their symptoms.
Demographic, medical, and social histories were obtained by standardized interviews of parents/guardians. Clinical laboratory evaluation, initial presentation, hospital course, underlying conditions and discharge diagnoses were obtained from medical records. Prematurity was defined as parental report of the child being born ≥4 weeks early. Stool specimens were collected from each enrolled child within 14 days of symptom onset and were tested using a commercial enzyme immunoassay (Rotaclone®). Only children with laboratory-confirmed rotavirus infection were included in this analysis.
For each laboratory-confirmed rotavirus-positive child, hospital and ED billing costs and physician charge data were gathered from accounting databases at each site. To ensure that children who had multiple encounters with providers were captured in the cost data, ED visits and hospitalizations that occurred within 72 h of the enrollment visit and had ICD-9 codes consistent with acute gastroenteritis were included. Children admitted to the hospital through the ED were categorized as hospitalized. Children were classified into three groups: inpatient hospitalization, short-stay admission, or ED visit. The short-stay patients were children admitted for observation whose stay was generally <24 h. Costs were sorted into five summary categories: diagnostics, therapeutics, room costs, medical supplies and physician services [23]. Since cost data were not available for physician services, charges were used. All costs were converted to 2009 dollars using the Medical Consumer Price Index.
The medical records of all hospitalized children were reviewed by an investigator at each site (MAS, GAW, KME) to examine factors that incurred the highest costs. Records were reviewed for underlying medical conditions or an atypical initial clinical presentation for acute gastroenteritis. An atypical presentation was defined as altered mental status, findings suggesting sepsis, shock, or an intra-abdominal process (including appendicitis and intussusception).
The Institutional Review Boards at the CDC and each participating NVSN site approved the study. Informed written consent was obtained from the parents/guardians of enrolled children.
2.2. Statistical analyses
To determine medical costs, we used univariate analysis and calculated means, standard deviations, medians, and 5–95th percentiles. Chi-square and Fisher's exact tests were used to analyze the relationships and characteristics associated with patient location. The Wilcoxon rank-sum and Kruskal–Wallis tests were used to determine group differences in the median total costs and length of hospital stays. A multivariate semi-log linear regression analysis was conducted to determine factors associated with increased costs. Variations in the logarithm costs of a rotavirus-associated hospitalization or short-stay visit were examined in relation to the relative variability of independent variables such as atypical presentations, underlying conditions, ICU requirement, age <3 months, recruitment site, and health insurance type. A two-sided p-value of <0.05 was considered statistically significant. All statistical analyses were conducted in SAS version 9.3 (SAS Institute Inc., Cary, NC).
The total costs of medically attended rotavirus (hospitalizations and ED visits) were estimated using median and mean costs from this study and rates of hospitalizations and ED visits from our population-based surveillance previously published for 2006–2008 [20–22]. These same methods were used to calculate the 2009 rates [20–22]. Rate estimates were calculated using the weighted number of laboratory-confirmed rotavirus hospitalizations divided by the number of children within the age cohort in the county population, as determined by the census. Weighting was done to account for the number of surveillance days, the proportion of the eligible children enrolled, and the percentage of stool samples collected. For the 4 year time period, 936 hospitalized children were eligible; 806 (86%) were enrolled; 702 (87%) had a stool specimen and 697 (99%) stool specimens were tested. For the emergency department, there were 1914 children eligible; 1526 (80%) were enrolled; 971 (64%) submitted a stool specimen and 967 (99.6%) stools were tested. To estimate the yearly US rotavirus disease burden, the yearly rotavirus rates (hospitalizations or ED) were multiplied by the total number of children residing in the US according to the 2000 US Census estimate [10]. To estimate the cost burden, the national rotavirus rates were multiplied by the median cost per child.
3. Results
During the four study seasons, 583 laboratory-confirmed rotavirus cases were detected in the hospital and ED settings; 278 from Cincinnati, 131 from Nashville, and 174 from Rochester. Cost data were not available for 15 cases: 12 cases from Nashville (7 inpatient and 5 ED) and 3 inpatient cases from Rochester. Deviations from the protocol resulted in the exclusion of 16 ED cases from Cincinnati. Of the 552 remaining rotavirus cases, 276 were inpatients, of whom 113 (41%) were short-stay admissions, and 276 were seen only in the ED. The characteristics for children hospitalized, admitted for short-stay, or seen in the ED are shown in Table 1. A significant difference existed overall among the 3 groups for race (p < 0.001), and insurance status (p < 0.001). ED patients were significantly more likely to have public or no insurance than children hospitalized or admitted for a short-stay visit (p = 0.007). ED patients were significantly less likely to be non-Hispanic white compared to children hospitalized or admitted for a short-stay visit (p < 0.001). Significant differences existed in age overall among the three groups (p < 0.001). ED children and those admitted for a short stay admission were more likely to be ≥3 months of age compared to hospitalized children (p < 0.001).
Table 1.
Characteristics of children with rotavirus-associated hospital, short-stay and emergency department visits, 2006-2009.
| Total N = 552 | Hospitalized n = 163 (30%) n (%) |
Short stay n = 113 (20%) n (%) |
Emergency department n = 276 (50%) n (%) |
p-Value |
|---|---|---|---|---|
| Gender | ||||
| Male | 76 (47) | 55 (49) | 151 (55) | 0.22 |
| Female | 87 (53) | 58 (51) | 125 (45) | |
| Ethnicity/racea | ||||
| White/non-Hispanic | 91 (56) | 69 (62) | 105 (38) | <0.001 |
| Black/non-Hispanic | 36 (22) | 34 (30) | 99 (36) | |
| Other | 35 (22) | 9(8) | 69 (25) | |
| Insuranceb | ||||
| Private | 76 (47) | 61 (54) | 105 (38) | 0.02 |
| Public | 79 (49) | 42 (37) | 143(52) | |
| None | 7 (4) | 10(9) | 26 (9) | |
| Age in months | ||||
| <3 | 24 (15) | 4 (4) | 7 (3) | <0.001 |
| 3–5 | 4(2) | 12 (11) | 20 (7) | |
| 6–11 | 22 (13) | 18 (16) | 62 (22) | |
| 12–17 | 40 (25) | 27 (24) | 64 (23) | |
| 18–23 | 34 (21) | 21 (19) | 66 (24) | |
| 24–35 | 39 (24) | 31 (27) | 57 (21) |
Bold values indicate a statistically significant difference between columns.
5 children's race is unknown (1 inpatient, 1 short-stay patient and 3 ED patients).
3 children's insurance type is unknown (1 inpatient and 2 ED patients).
3.1. Hospitalization and Short Stay Costs Associated with Rotavirus Disease
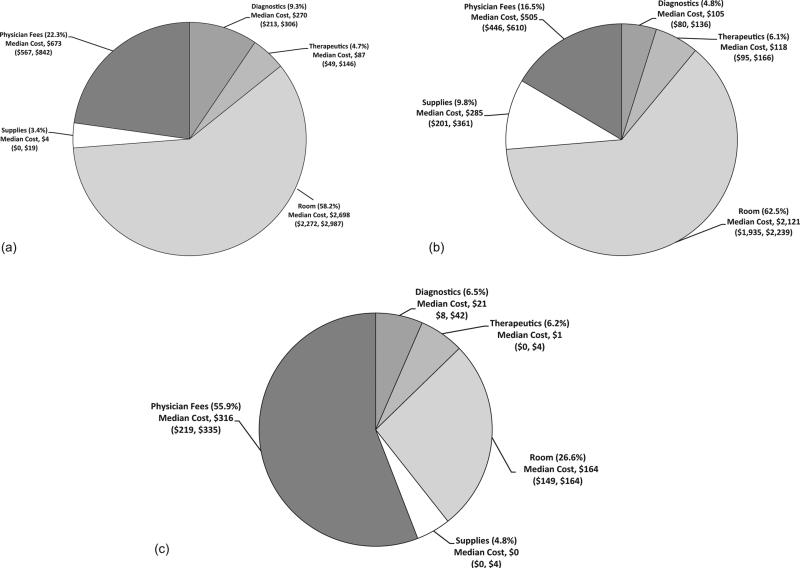
The overall mean medical cost per rotavirus–associated hospitalization and short-stay admission was $5011, and $3646 respectively with a median cost of $3915 and $3474 respectively (Fig. 1a and b). The combined hospitalization and short-stay mean cost was $4452 and the median cost was $3712. Of hospitalization costs, 58% of the total medical costs were due to hospital and ED room costs. Physician fees accounted for another 22% of the total hospital costs. Of short-stay admissions, room charges accounted for 62% of total costs and physician fees were 16% of total costs. More detailed hospitalization and short-stay cost data can be found in Supplemental Table 1 with site-specific data in Supplemental Table 2.
Fig. 1.
(a) Medical costs for rotavirus-associated hospitalizations proportion and median costs (95% confidence intervals) by cost category overall median cost for rotavirus-associated hospitalization: $3915 ($3655, $4340). (b) Medical costs for rotavirus-associated short-stay visits proportion and median costs (95% confidence intervals) by cost category overall median cost for rotavirus-associated short-stay visit: $3474 ($3655, $4340). (c) Medical costs for rotavirus-associated emergency department visits proportion and median costs (95% confidence intervals) by cost category overall median cost for rotavirus-associated ED visits: $630 ($575, $690).
3.2. Emergency department visit costs associated with rotavirus disease
The overall mean medical cost per rotavirus-associated ED visit was $810 with a median cost of $630. The majority of the costs were from room (27%) and physician fees (56%) while diagnostics and therapeutics represented only a small proportion (7% and 6% respectively) (Fig. 1c). More detailed site-specific ED cost data can be found in Supplemental Table 3.
3.3. Diagnostic testing and antibiotic use (Table 2)
Table 2.
Overall proportion of children by visit type with antibiotic use, renal panel, radiographs and rotavirus testing.
| Hospitalized N =163 |
Short-stay N=113 |
ED N=276 |
||||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Antibioticsa,b,c | 50 | 31 | 20 | 18 | 11 | 4 |
| Renal paneld,e | 143 | 88 | 92 | 81 | 86 | 31 |
| Radiographf,g | 50 | 31 | 29 | 26 | 27 | 10 |
| Rotavirus testh,i,j | 79 | 48 | 26 | 23 | 8 | 3 |
Hospitalized vs. short stay p-value = 0.01.
Hospitalized vs. ED p-value < 0.001.
Short-stay vs. ED p-value < 0.001.
Hospitalized vs. ED p-value < 0.001.
Short-stay vs. ED p-value < 0.001.
Hospitalized vs. ED p-value < 0.001.
Short-stay vs. ED p-value < 0.001.
Hospitalized vs. short stay p-value < 0.001.
Hospitalized vs. ED p-value < 0.001.
Short-stay vs. ED p-value < 0.001.
Overall, 31% of hospitalized children were treated with antibiotics compared to 18% of short-stay patients and 4% of ED patients. Of patients hospitalized, 55% received intravenous antimicrobial therapy compared to 23% of short-stay and 5% of ED patients (p < 0.001).
Renal panels were frequently used for evaluating children hospitalized (88%) or admitted for a short-stay (81%) compared to ED patients (31%). Similarly, radiologic evaluations were used for 31%, 26% and 10% of children hospitalized, with short stay visits or with ED visits, respectively. Overall, rotavirus testing was infrequently ordered for children hospitalized (48%) or admitted for a short stay (23%) and even less frequently requested for ED patients (3%).
3.4. Factors associated with high costs
Factors examined with increased costs for the 276 hospitalized and short-stay admissions are shown in Table 3. As expected, an ICU stay generated significantly greater costs than a non-ICU stay (median cost of $13,321 versus $3657 respectively). While children with an ICU stay had higher individual costs, children without ICU care accounted for 91% of the total costs and 93% of the total bed days. Medical costs were significantly affected by age (p = 0.001). Children <3 months of age had significantly higher costs (p < 0.001) compared to older children and accounted for 10% of the rotavirus-related hospitalizations and 16% of the total costs. In the univariate analysis, increased costs were significantly associated with an ICU admission (p = 0.008), race other than white/non-Hispanic (p = 0.04), insurance status (p = 0.02), young age (p = 0.001), an atypical presentation (p < 0.001) and study site (p < 0.001). In the multivariate regression analysis, factors significantly associated with higher costs included atypical presentation (p < 0.001), presence of an underlying condition (p = 0.003), age <3 months (p = 0.02) and study site (p < 0.001). For those children with an atypical presentation, the incremental cost was $1861 compared to those with a typical presentation. Children with an underlying condition had an incremental cost of $1168 compared to those without. Children <3 months of age had an incremental cost of $1144 compared to those ≥3 months of age. Costs were $2585 less for children from Nashville compared to children from Cincinnati (p < 0.001) and $2408 less for children from Nashville compared to Rochester (p < 0.001).
Table 3.
Univariate analysis of estimated medical costs and LOS for combined rotavirus-associated hospitalizations and short-stay visits, 2006–2009.
| Hospital costs |
Length of stay |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean | Median | 5-95th percentiles | p-Value | Percent of cost | N | Mean | Median | Range | p-Value | Total bed days | Percent total bed days | |
| ICU | |||||||||||||
| No | 268 | $4180 | $3657 | (1430–8085) | 0.008 | 91% | 268 | 2 | 1 | (0–12) | 0.002 | 424 | 93% |
| Yes | 8 | $13,566 | $13,321 | (5598–22,631) | 9% | 8 | 4 | 3 | (2–7) | 30 | 7% | ||
| Prematurity | |||||||||||||
| No | 252 | $4465 | $3712 | (1691–9180) | 0.99 | 92% | 252 | 2 | 1 | (0–12) | 0.46 | 418 | 92% |
| Yes | 24 | $4316 | $3614 | (1200–7872) | 8% | 24 | 2 | 1 | (0–5) | 36 | 8% | ||
| Gender | |||||||||||||
| Male | 131 | $4272 | $3708 | (1248–8533) | 0.92 | 46% | 131 | 2 | 1 | (0–6) | 0.63 | 213 | 47% |
| Female | 145 | $4614 | $3752 | (1762–9411) | 54% | 145 | 2 | 1 | (0–12) | 241 | 53% | ||
| Racea | |||||||||||||
| Other | 114 | $4863 | $3939 | (1430–10,924) | 0.04 | 45% | 114 | 2 | 2 | (0–7) | 0.02 | 207 | 46% |
| White/non-Hispanic | 160 | $4194 | $3512 | (1568–7759) | 55% | 160 | 2 | 1 | (0–12) | 245 | 54% | ||
| Private insuranceb | |||||||||||||
| No | 138 | $4837 | $3954 | (1353–10,511) | 0.02 | 54% | 138 | 2 | 1 | (0–12) | 0.16 | 244 | 54% |
| Yes | 137 | $4071 | $3543 | (1445–8473) | 46% | 137 | 2 | 1 | (0–6) | 209 | 46% | ||
| Age group (months) | |||||||||||||
| <3 | 28 | $7154 | $5227 | (2429–22,631) | 0.001 | 16% | 28 | 3 | 2 | (0–12) | 0.03 | 71 | 16% |
| 3–5 | 16 | $4603 | $4513 | (1114–9650) | 6% | 16 | 2 | 1 | (0–6) | 28 | 6% | ||
| 6–11 | 40 | $4160 | $3751 | (1446–8137) | 14% | 40 | 2 | 2 | (0–4) | 65 | 14% | ||
| 12–17 | 67 | $3745 | $3434 | (1248–6905) | 20% | 67 | 1 | 1 | (0–5) | 99 | 22% | ||
| 18–23 | 55 | $4528 | $3509 | (1445–12,400) | 20% | 55 | 2 | 1 | (0–6) | 86 | 19% | ||
| 24–35 | 70 | $4121 | $3624 | (1820–8533) | 23% | 70 | 2 | 1 | (0–6) | 105 | 23% | ||
| Underlying condition | |||||||||||||
| No | 238 | $4268 | $3657 | (1430–8473) | 0.16 | 83% | 238 | 2 | 1 | (0–12) | 0.13 | 369 | 81% |
| Yes | 38 | $5606 | $4440 | (733–19,277) | 17% | 38 | 2 | 2 | (0–7) | 85 | 19% | ||
| Atypical presentation | |||||||||||||
| No | 227 | $3838 | $3474 | (1353–7201) | <0.001 | 71% | 227 | 1 | 1 | (0–7) | 0.006 | 333 | 73% |
| Yes | 49 | $7296 | $5491 | (2582–19,277) | 29% | 49 | 2 | 2 | (0–12) | 121 | 27% | ||
| Site | |||||||||||||
| Cincinnati | 118 | $5255 | $4239 | (2290–10,924) | <0.001 | 50% | 118 | 2 | 1 | (0–12) | <0.001 | 190 | 42% |
| Nashville | 62 | $2700 | $2581 | (746–5112) | 14% | 62 | 1 | 1 | (0–4) | 75 | 17% | ||
| Rochester | 96 | $4597 | $3835 | (2277–9634) | 36% | 96 | 2 | 2 | (0–7) | 189 | 42% | ||
Bold values indicate a statistically significant difference between categories.
2 children are unknown for race.
1 child is unknown for insurance.
3.5. Projected national costs associated with rotavirus disease
The yearly rates of hospitalizations, median and mean costs and the estimated national costs are shown in Table 4. The combined 2006 and 2007, pre-vaccine rate of rotavirus-associated hospitalizations and short-stay visits was 22.1 per 10,000 [8] and the median cost per hospitalized child was $3581 (95% CI $3328–3802). Using this rotavirus hospitalization rate and the median cost per hospitalization and extrapolating to the US population of children <3 years of age [10], for the 2006 and 2007 seasons, we estimated 25,370 rotavirus-related hospitalizations occurred annually with $91 million (95% CI $78–104 million) in medical costs in 2009 US dollars. In the 2008 and 2009 post-vaccine seasons, the average rate of hospitalization was 6.3/10,000 and the median cost was $4304 (95% CI $3797–5018). We estimated there were 7191 hospitalizations due to rotavirus each year at a cost of $31 million (95% CI $21–42 million). Comparing the pre- and post-vaccine periods, after implementing the rotavirus immunization program in the US, there was a decrease of $120 million in costs for rotavirus-associated hospitalizations for the two-year period.
Table 4.
(a) Estimated national annual costs for rotavirus hospitalizations in the United States, (b) Estimated national annual costs for emergency department (ED) visits in the United States.
| (a) Hospitalizations | ||||||
|---|---|---|---|---|---|---|
| Season | Rate per 10,000 | Number of hospitalizations | Median cost | Hospital costs (95% CI) | Mean cost | Hospital costs (95% CI) |
| 2006 | 22.5 (19.2–25.7) | 25,887 | S3592 | $92,981,170 ($79,343,932, $106,205,159) | $4159 | $107,665,394 ($91,874,470, $122,977,806) |
| 2007 | 21.6 (18.5–24.6) | 24,852 | $3565 | $88,595,869 ($75,880,722, $100,900,851) | $4257 | $105,794,258 ($90,610,823, $120,487,905) |
| 2008 | 2.4 (1.0–4.1) | 2761 | $4297 | $11,866,135 ($4944,223, $20,271,314) | $4651 | $12,842,876 ($5,351,198, $21,939,913) |
| 2009 | 10.1 (7.6–12.7) | 11,621 | $4311 | $50,091,588 ($37,692,680, $62,986,453) | $5346 | $62,123,373 ($46,746,300, $78,115,528) |
| (b) Emergency department visits | ||||||
|---|---|---|---|---|---|---|
| Season | Rate per 10,000 | Number of ED visits | Median cost (95% CI) | ED costs | Mean cost (95% CI) | ED costs |
| 2006 | 301.0 | 346,315 | $543 ($379, $575) | $187,920,653 | $586 ($452, $721) | $202,940,538 |
| 2007 | 281.0 | 323,304 | $724 ($554, $823) | $234,105,557 | $886 ($765–$1006) | $286,447,303 |
| 2008 | 44.2 | 50,854 | $540 ($503, $873) | $27,469,886 | $628 ($470, $786) | $31,936,448 |
| 2009 | 97.8 | 112,524 | $827 ($672, $961) | $93,015,369 | $992 ($834, $1150) | $111,623,394 |
For ED visits, in the pre-vaccine period, the overall median cost was $574 (95% CI $539–645). Using the 2006 and 2007 ED visit rate of 291/10,000, the median costs of an ED visit and extrapolating to the US population of children <3 years of age [24], there were an estimated 334,810 rotavirus-related ED visits annually with estimated annual medical costs of $192 million in 2009 US dollars. In the post-vaccine period, the average annual rotavirus-associated ED visit rate was 71 per 10,000 and the median cost per child per ED visit was $794 (95% CI $669–909) with an estimated annual cost of $65 million. Comparing the pre- and post-vaccine periods, there was a decrease of $254 million in costs for rotavirus-associated ED visits for the two-year period.
4. Discussion
To our knowledge, this is the first population-based study utilizing active surveillance with laboratory-confirmed rotavirus cases and billing data to determine medical costs associated with rotavirus-associated hospitalizations and ED visits. Our national estimate of rotavirus hospitalization costs of $93 million in 2006 (adjusted to 2009 dollars) is less than previously reported unadjusted costs of $150–198 million [1–3] and is far less than the adjusted costs of $190–289 million. This difference is primarily due to the lower national estimate of 25,887 rotavirus hospitalizations in our study compared with previous estimates of 55,000–70,000. The differences in our direct estimates and previously published indirect estimates are likely due to the methods used for identifying children with rotavirus. The indirect method estimated more children hospitalized with rotavirus compared to our direct method. This could be due to including children who developed diarrhea after their admission or by overestimating the proportion of acute gastroenteritis cases due to rotavirus. For children seen in the ED, the indirect method likely underestimates ED-associated rotavirus visits since diagnoses such as dehydration, vomiting and viral illness are not included for identification of children with acute gastroenteritis. In addition, the indirect estimate used a much lower proportion of rotavirus-positive acute gastroenteritis compared to active surveillance estimates with laboratory-confirmed rotavirus, thus underestimating the total number of rotavirus-associated ED visits. Our pre-licensure median cost of ~$3581 was higher than the median cost of $2962 in the Widdowson study [3]. The costs for the Widdowson study were based on a two day hospitalization for rotavirus gastroenteritis using the rotavirus-specific ICD-9-CM code [3] while our costs were based on actual costs of children hospitalized for rotavirus. Another potential reason for the differences is that our surveillance program identified children presenting with symptoms of acute gastroenteritis and did not include children with nosocomial rotavirus infections, which may represent up to 20% of all rotavirus cases in hospitalized children [25]. A simple inflation of our costs by 20% to account for the number of children with nosocomial infections is unlikely to accurately reflect the total costs attributed to rotavirus in this group of children with nosocomial disease since these children may have more complex medical issues and higher costs for their rotavirus-associated illness. With that said, if we use the same median costs/case as our community-associated cases and assume a 20% increase in the number of hospitalizations, the average pre-licensure cost per year would be $108,946,117. In addition, our study included children <3 years of age while other studies included children <5 years of age. If we conservatively increase the number of children hospitalized by 10% to account for this broader age range in addition to the increase for nosocomial infections, the cost per year pre-licensure, would be $118,024,960. If there was a 40% increase due to nosocomial cases and age differences, the average costs per year pre-licensure would be $127,103,803. We included short-stay admissions as hospitalizations while administrative database studies include short-stay visits as ED visits. Short-stay visits increased the number of hospitalizations in our estimates, but these admissions did have a lower median cost compared to hospitalized children. Since national estimates of the proportion of children cared for as short-stay admissions is not known, it is difficult to assess how this would affect estimates from administrative databases.
Since the implementation of the rotavirus vaccine program, we demonstrated a 66% decrease in the costs of US rotavirus hospitalizations. Similarly, another study examining decreased rotavirus vaccine and health care utilization for diarrhea in US children <5 years of age using MarketScan data, found a reduction of 64,855 hospitalizations due to diarrhea (not specifically rotavirus) in the 2007–2009 time period with a savings of $278 million in treatment costs [13]. Comparing these estimates directly is difficult given methodological differences. However, both studies, found a dramatic decrease in diarrhea-associated hospitalization costs after rotavirus vaccine introduction. Given the post-licensure, biennial rotavirus activity seen in our study and others [13,26,27], it will be important to take this into account when examining the economic impact of the rotavirus vaccine program.
In our study, we found higher cost hospitalizations were significantly associated with study site, younger age (<3 months), atypical clinical presentation, and presence of an underlying condition. By using sensitive, rapid and effective techniques for evaluation and diagnosis of rotavirus there could be further cost-savings by reducing unnecessary testing and antibiotic use.
In contrast to the pre-vaccine hospitalization estimates, our national ED cost estimates of $192 million annually were significantly higher than previous unadjusted estimates of $37–71 million, even after adjusting for 2009 dollars ($61–86 million) [1–3]. This difference was driven by both higher estimates of ED visits and costs in our study compared with previous studies. Prior studies estimated 20–28% of ED visits for diarrhea were due to rotavirus. However, our study found 37–49% of children with acute gastroenteritis in the ED have rotavirus [17,19,20,22]. In addition, the Widdowson study used a median cost of $332 which was much lower than our pre-licensure median ED cost of $574. This may be due to the use of diarrhea-specific ICD-9 codes to determine the ED costs [1–3]. These codes do not include codes for vomiting, dehydration or viral illness, which could result in not only an underestimation of acute gastroenteritis cases but also more severe cases of acute gastroenteritis with significant dehydration. There could also be shifts from inpatient to ED management which would also increase the number of ED visits and decrease the number of hospitalizations compared to past years [28]. In our study, we did see a significantly higher median cost for ED cases post-licensure compared to pre-licensure ED costs.
Among the limitations of our study is that despite conducting population-based surveillance for four seasons at three sites, our study was limited to community-acquired rotavirus in immuno-competent children in urban areas. Our results are likely to be representative of similarly sized counties in the United States. Our study also used unadjusted physician charges, which may overestimate total medical costs by approximately 6–14% [29]. Lastly, a possible limitation is that 16 cases were excluded for protocol deviations from Cincinnati and 15 cases from the other sites for missing cost data. Most of these children were ED cases so there was little variation in their costs compared to cases with available data. For hospitalized children with missing data, the lengths of stay for Cincinnati and Rochester inpatients were not significantly different compared to those included from their respective sites. However, the 7 hospitalized children from Nashville with missing data had a significantly longer length of stay compared to those in the study. Not including these children may have contributed to the overall lower costs for Nashville.
5. Conclusions
Costs for rotavirus hospitalizations and ED visits accounted for a significant economic burden in the US. Although our cost estimates for hospitalizations were lower compared to previous studies, our ED visit costs were much higher than previously estimated. There has been a dramatic decline in rotavirus-associated hospitalization and ED visit costs since the widespread use of rotavirus vaccine in the US with an estimated annual savings of $187 million dollars in health care costs.
Supplementary Material
Acknowledgements
Cincinnati: Jessica Schloemer, Michol Holloway and Mary Sandquist for coordination of the studies, Michelle Roth, Vanessa Florian, Ben Sillies, Nancy Back for enrollment of children, Sahle Amsulu for data management, and Douglas Knowlton for laboratory testing.
Nashville: Diane Kent for coordination of the study, Erin Keckley, Mariah Dailey, Carol Ann Clay, Carolyn Cooper, and Angela Ibarra for enrollment of children, Yuwei Zhu for data management, and Jody Peters for laboratory testing.
Rochester: Geraldine Lofthus and Christina Albertin for study coordination, Linda Anderson, Nancy Jenks, and Charlene Freundlich for enrollment of children; Kenneth Schnabel and Lynne Shelley for laboratory testing.
Centers for Disease Control and Prevention: Haley Clayton for study coordination, Minnie Wang for data management and Elizabeth Teel for laboratory testing.
Footnotes
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.vaccine.2013.06.085.
References
- 1.Tucker AW, Haddix AC, Bresee JS, Holman RC, Parashar UD, Glass RI. Cost-effectiveness analysis of a rotavirus immunization program for the United States. JAMA. May 6. 1998;279(17):1371–6. doi: 10.1001/jama.279.17.1371. [DOI] [PubMed] [Google Scholar]
- 2.Zimmerman CM, Bresee JS, Parashar UD, Riggs TL, Holman RC, Glass RI. Cost of diarrhea-associated hospitalizations and outpatient visits in an insured population of young children in the United States. Pediatr Infect Dis J. 2001 Jan;20(1):14–9. doi: 10.1097/00006454-200101000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Widdowson MA, Meltzer MI, Zhang X, Bresee JS, Parashar UD, Glass RI. Cost-effectiveness and potential impact of rotavirus vaccination in the United States. Pediatrics. Apr. 2007;119(4):684–97. doi: 10.1542/peds.2006-2876. [DOI] [PubMed] [Google Scholar]
- 4.Cortese MM, Parashar UD. Prevention of rotavirus gastroenteritis among infants and children: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2009 Feb;58(RR-2):1–25. [PubMed] [Google Scholar]
- 5.Ho MS, Glass RI, Pinsky PF, Anderson LJ. Rotavirus as a cause of diarrheal morbidity and mortality in the United States. J Infect Dis. 1988 Nov;158(5):1112–6. doi: 10.1093/infdis/158.5.1112. [DOI] [PubMed] [Google Scholar]
- 6.Jin S, Kilgore PE, Holman RC, Clarke MJ, Gangarosa EJ, Glass RI. Trends in hospitalizations for diarrhea in United States children from 1979 through 1992: estimates of the morbidity associated with rotavirus. Pediatr Infect Dis J. 1996 May;15(5):397–404. doi: 10.1097/00006454-199605000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Parashar UD, Holman RC, Clarke MJ, Bresee JS, Glass RI. Hospitalizations associated with rotavirus diarrhea in the United States, 1993 through 1995: surveillance based on the new ICD-9-CM rotavirus-specific diagnostic code. J Infect Dis. 1998 Jan;177(1):13–7. doi: 10.1086/513808. [DOI] [PubMed] [Google Scholar]
- 8.Chang HG, Glass RI, Smith PF, Cicirello HG, Holman RC, Morse DL. Disease burden and risk factors for hospitalizations associated with rotavirus infection among children in New York State, 1989 through 2000. Pediatr Infect Dis J. 2003 Sep;22(9):808–14. doi: 10.1097/01.inf.0000086404.31634.04. [DOI] [PubMed] [Google Scholar]
- 9.Chang HG, Smith PF, Tserenpuntsag B, Markey K, Parashar U, Morse DL. Reduction in hospitalizations for diarrhea and rotavirus infections in New York state following introduction of rotavirus vaccine. Vaccine. 2010 Jan;28(3):754–8. doi: 10.1016/j.vaccine.2009.10.075. [DOI] [PubMed] [Google Scholar]
- 10.Malek MA, Curns AT, Holman RC, Fischer TK, Bresee JS, Glass RI, et al. Diarrhea- and rotavirus-associated hospitalizations among children less than 5 years of age: United States, 1997 and 2000. Pediatrics. 2006 Jun;117(6):1887–92. doi: 10.1542/peds.2005-2351. [DOI] [PubMed] [Google Scholar]
- 11.Fischer TK, Viboud C, Parashar U, Malek M, Steiner C, Glass R, et al. Hospitalizations and deaths from diarrhea and rotavirus among children <5 years of age in the United States, 1993–2003. J Infect Dis. 2007 Apr;195(8):1117–25. doi: 10.1086/512863. [DOI] [PubMed] [Google Scholar]
- 12.Ma L, El Khoury AC, Itzler RF. The burden of rotavirus hospitalizations among Medicaid and non-Medicaid children younger than 5 years old. Am J Public Health. 2009 Oct;(Suppl 2):S398–404. doi: 10.2105/AJPH.2008.148494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cortes JE, Curns AT, Tate JE, Cortese MM, Patel MM, Zhou F, et al. Rotavirus vaccine and health care utilization for diarrhea in U.S. children. N Engl J Med. 2011 Sep;365(12):1108–17. doi: 10.1056/NEJMoa1000446. [DOI] [PubMed] [Google Scholar]
- 14.Charles MD, Holman RC, Curns AT, Parashar UD, Glass RI, Bresee JS. Hospitalizations associated with rotavirus gastroenteritis in the United States, 1993-2002. Pediatr Infect Dis J. 2006 Jun;25(6):489–93. doi: 10.1097/01.inf.0000215234.91997.21. [DOI] [PubMed] [Google Scholar]
- 15.Brandt CD, Kim HW, Rodriguez WJ, Arrobio JO, Jeffries BC, Stallings EP, et al. Pediatric viral gastroenteritis during eight years of study. J Clin Microbiol. 1983 Jul;18(1):71–8. doi: 10.1128/jcm.18.1.71-78.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsu VP, Staat MA, Roberts N, Thieman C, Bernstein DI, Bresee J, et al. Use of active surveillance to validate international classification of diseases code estimates of rotavirus hospitalizations in children. Pediatrics. 2005 Jan;115(1):78–82. doi: 10.1542/peds.2004-0860. [DOI] [PubMed] [Google Scholar]
- 17.Mast TC, Walter EB, Bulotsky M, Khawaja SS, DiStefano DJ, Sandquist MK, et al. Burden of childhood rotavirus disease on health systems in the United States. Pediatr Infect Dis J. 2010 Feb;29(2):e19–25. doi: 10.1097/inf.0b013e3181ca7e2e. [DOI] [PubMed] [Google Scholar]
- 18.Staat MA, Azimi PH, Berke T, Roberts N, Bernstein DI, Ward RL, et al. Clinical presentations of rotavirus infection among hospitalized children. Pediatr Infect Dis J. 2002 Mar;21(3):221–7. doi: 10.1097/00006454-200203000-00012. [DOI] [PubMed] [Google Scholar]
- 19.Yee EL, Staat MA, Azimi P, Bernstein DI, Ward RL, Schubert C, et al. Burden of rotavirus disease among children visiting pediatric emergency departments in Cincinnati, Ohio, and Oakland, California, in 1999–2000. Pediatrics. 2008 Nov;122(5):971–7. doi: 10.1542/peds.2007-1609. [DOI] [PubMed] [Google Scholar]
- 20.Payne DC, Staat MA, Edwards KM, Szilagyi PG, Gentsch JR, Stockman LJ, et al. Active, population-based surveillance for severe rotavirus gastroenteritis in children in the United States. Pediatrics. 2008 Dec;122(6):1235–43. doi: 10.1542/peds.2007-3378. [DOI] [PubMed] [Google Scholar]
- 21.Payne DC, Staat MA, Edwards KM, Szilagyi PG, Weinberg GA, Hall CB, et al. Direct and indirect effects of rotavirus vaccination upon childhood hospitalizations in 3 US counties, 2006–2009. Clin Infect Dis. 2011 Aug;53(3):245–53. doi: 10.1093/cid/cir307. [DOI] [PubMed] [Google Scholar]
- 22.Payne DC, Szilagyi PG, Staat MA, Edwards KM, Gentsch JR, Weinberg GA, et al. Secular variation in United States rotavirus disease rates and serotypes: implications for assessing the rotavirus vaccination program. Pediatr Infect Dis J. 2009 Nov;28(11):948–53. doi: 10.1097/INF.0b013e3181a6ad6e. [DOI] [PubMed] [Google Scholar]
- 23.Keren R, Zaoutis TE, Saddlemire S, Luan XQ, Coffin SE. Direct medical cost of influenza-related hospitalizations in children. Pediatrics. 2006 Nov;118(5):e1321–7. doi: 10.1542/peds.2006-0598. [DOI] [PubMed] [Google Scholar]
- 24.US Census Bureau [4.1.2010];American Fact Finder. 2000 Available from: http://factfinder.census.gov/servlet/DTTable?_bm=y&-ds_name=DEC_2000_SF1_U&-CONTEXT=dt&-mt_name=DEC_2000_SF1_U_P012&-redoLog=false&-_caller=geoselect&-geo_id=01000US&-geo_id=NBSP&-format=&-_lang=en.
- 25.Smith MJ, Clark HF, Lawley D, Bell LM, Hodinka RL, DiStefano DJ, et al. The clinical and molecular epidemiology of community- and healthcare-acquired rotavirus gastroenteritis. Pediatr Infect Dis J. 2008 Jan;27(1):54–8. doi: 10.1097/INF.0b013e31814b279d. [DOI] [PubMed] [Google Scholar]
- 26.Centers for Disease Control and Prevention [19.4.2013];National Respiratory and Enteric Virus Surveillance System (NREVSS) 2013 Available from: http://www.cdc.gov/surveillance/nrevss/
- 27.Desai R, Curns AT, Steiner CA, Tate JE, Patel MM, Parashar UD. All-cause gastroenteritis and rotavirus-coded hospitalizations among US children, 2000–2009. Clin Infect Dis. 2012 Aug;55(4):e28–34. doi: 10.1093/cid/cis443. [DOI] [PubMed] [Google Scholar]
- 28.Pont SJ, Carpenter LR, Griffin MR, Jones TF, Schaffner W, Dudley JA, et al. Trends in healthcare usage attributable to diarrhea, 1995–2004. J Pediatr. 2008 Dec;153(6):777–82. doi: 10.1016/j.jpeds.2008.06.037. [DOI] [PubMed] [Google Scholar]
- 29.Barbara O, Wynn MS. [6.1.2010];Evaluation of alternative methods to establish DRG relative weights. 2008 Available from: http://www.rand.org/pubs/working papers/WR560/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.