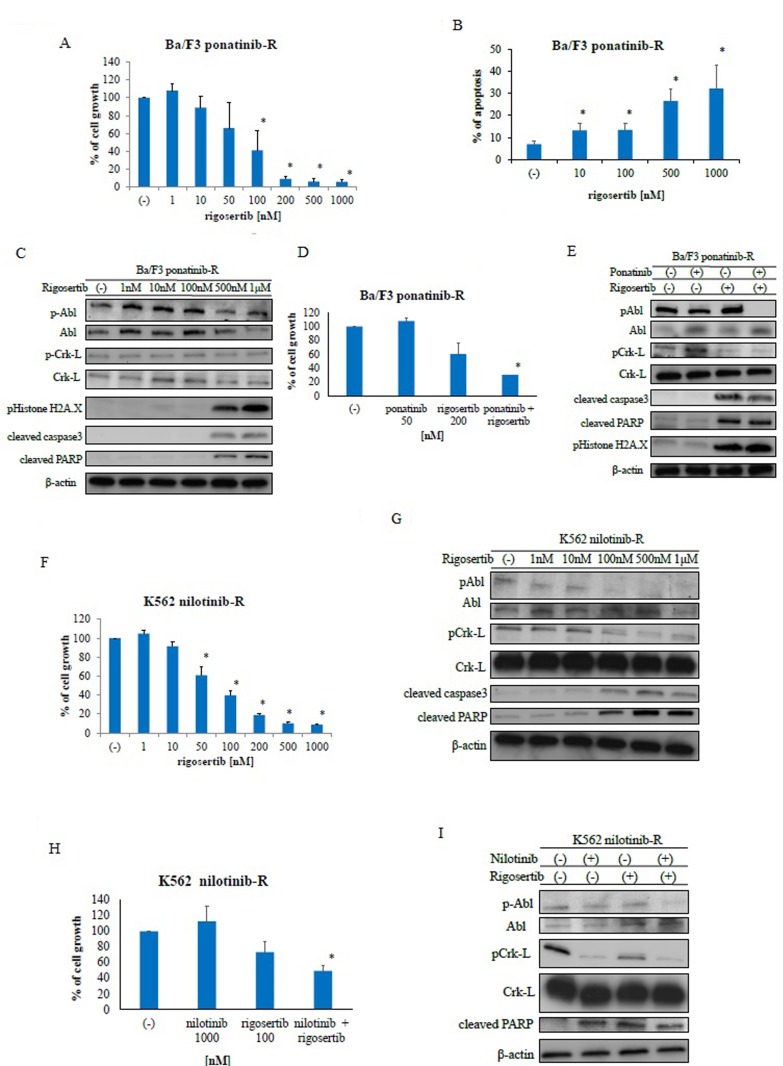
Figure 3. Rigosertib activity against Abelson tyrosine kinase inhibitor -resistant cells.
A., F. Cells were cultured in the presence or absence of rigosertib for 72 h. Viable cell numbers were counted; *P < 0.05 compared with the control. Results represent three independent experiments. B. Cells were treated with the indicated concentrations of rigosertib for 48 h. Percentages of apoptotic cells were determined; *P < 0.05 compared with the control. C., G. Cells were treated with rigosertib at the indicated concentrations for 24 h. Total extracts were examined via immunoblotting with anti-phospho ABL, phospho-Crk-L, phosphohistone H2A.X, cleaved caspase 3, cleaved-PARP, ABL, Crk-L, and β-actin antibodies. D., H. Cells were treated with the indicated concentrations of rigosertib or ponatinib, both, or nilotinib for 72 h. Percentages of cell growth were determined; *P < 0.05 compared with rigosertib treatment. E., I. Cells were treated with nilotinib or rigosertib, both, or ponatinib for 24 h. Total extracts were examined via immunoblotting with anti-phospho ABL, phospho-Crk-L, phosphohistone H2A.X, cleaved caspase 3, cleaved-PARP, ABL, Crk-L, and β-actin antibodies. ABL, Abelson; PARP, poly (ADP-ribose) polymerase.