Table 1.
Optimization of the reaction conditions.
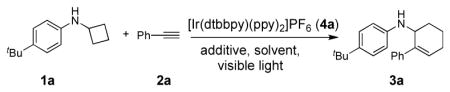
| ||||
|---|---|---|---|---|
| Entry[a] | Conditions | t [h] | Conv. of 1a [%][b] | Yield of 3a [%][b] |
| 1 | 4a (2 mol%), MeOH | 12 | 100 | 97 (90)[c] |
| 2 | 4a (2 mol%), MeOH, Air | 16 | 100 | 42 |
| 3 | without 4a, MeOH | 16 | 7 | 3 |
| 4 | 4a (2 mol%), MeOH, light bulb off | 16 | 10 | 7 |
| 5[d] | 4a (2 mol%), MeOH | 12 | 70 | 68 |
| 6[e] | 4a (2 mol%), MeOH | 12 | 29 | 27 |
Conditions: 1a (0.2 mmol, 0.1 M in degassed solvent), 2a (1 mmol), irradiation with two 18 W LED light bulbs at room temperature.
Yield determined by GC analysis using dodecane as an internal standard unless noted.
Isolated yields shown.
One 18 W LED light, reaction tube in a 55 °C water bath.
One 18 W LED light.
