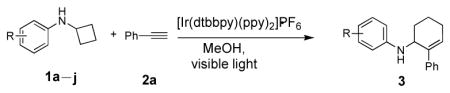
Table 2.
[4+2] Annulation of phenylacetylene (2a) with monocyclic cyclobutylanilines.
| ||||
|---|---|---|---|---|
| Entry[a] | Substrate | Product | t [h] | Yield[%][b] |
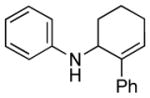
| 1 | 1b, R = H |
 3b |
12 | 76 |
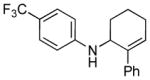
| 2 | 1c, R = 4-CF3 |
 3c |
24 | 79 |
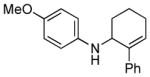
| 3 | 1d, R = 4-OMe |
 3d |
18 | 27 |
| 4 | 1e, R = 2-CN |
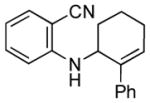 3e |
20 | 84 |
| 5 | 1f, R = 2-phenyl |
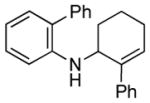 3f |
20 | 78 |
| 6 | 1g, R = 2-iPr |
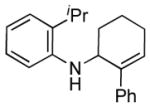 3g |
24 | 73 |
| 7 |
1h
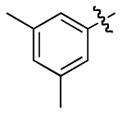
|
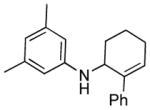 3h |
12 | 87 |
| 8 |
1i
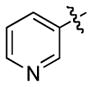
|
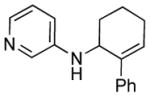 3i |
14 | 76 |
| 9 |
1j
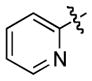
|
 3j |
18 | 83 |
Reaction condition: substrate (0.2 mmol, 0.1 M in degassed MeOH), 2a (1 mmol), 4a (2 mol%), irradiation with two 18 W LED light bulbs.
Yield of the isolated product.
