Table 3.
Scope of alkynes in the [4+2] annulation.
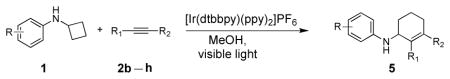
| |||||
|---|---|---|---|---|---|
| Entry[a] | Substrate | Alkyne | Product | t [h] | Yield[%][b] |
| 1 | 1a |
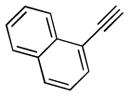 2b |
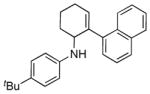 5a |
16 | 71 |
| 2[c] | 1a |
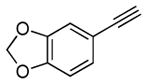 2c |
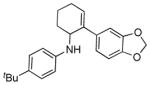 5b |
16 | 57 |
| 3 | 1j | 2b |
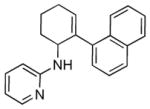 5c |
16 | 72 |
| 4 | 1f |
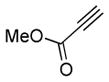 2d |
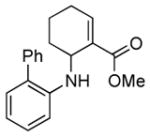 5d |
14 | 42 |
| 5 | 1a |
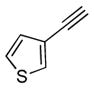 2e |
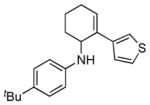 5e |
12 | 66 |
| 6 |
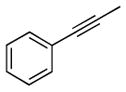 2f |
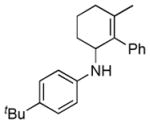 5f |
14 | 61 | |
| 7 | 1h |
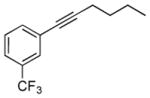 2g |
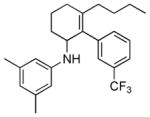 5g |
24 | 42 |
| 8 | 1c |
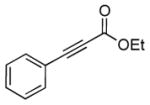 2h |
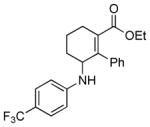 5h |
12 | 92 |
Reaction condition: substrate (0.2 mmol, 0.1 M in degassed MeOH), 2b–2h (0.6 mmol), 4a (2 mol%), irradiation with two 18 W LED light bulbs.
Yield of the isolated product.
Mixed solvent of MeOH and CH3NO2 (1:1).
