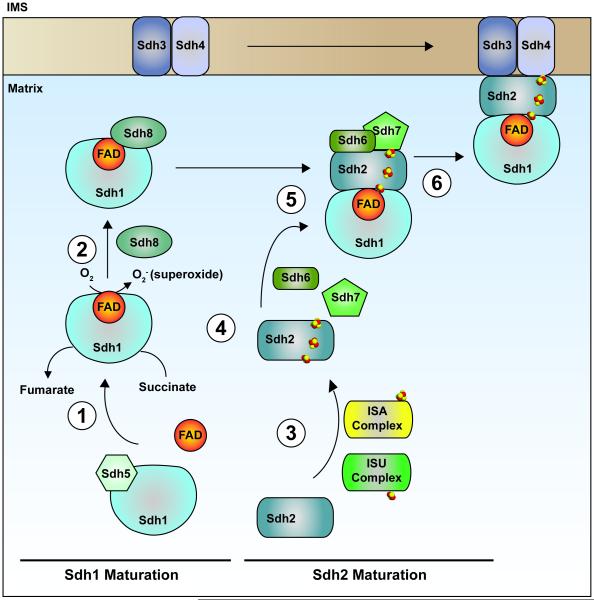
Figure 3. Model of the SDH assembly pathway.
Each SDH core subunit is translated in the cytosol and must be subsequently translocated to the mitochondria. Upon mitochondrial import, apo-Sdh1 is rapidly bound by the subunit-specific chaperone, Sdh5, forming a dimeric complex that supports covalent attachment of the FAD cofactor (1). Following covalent flavinylation, the Sdh1-Sdh5 dimer disintegrates resulting in a pool of flavinylated Sdh1 that is unbound by any other core subunits. This leads to the formation of a complex comprised of Sdh1 and Sdh8, another subunit-specific chaperone (2). The formation of this complex supports the formation of the subsequent Sdh1-Sdh2 soluble dimer and also prevents the spurious production of superoxide by flavinylated Sdh1. Meanwhile, apo-Sdh2 must also mature into a complex-competent subunit. This process involves the insertion of 3 Fe-S clusters generated by the ISU and ISA complexes. (3). Following maturation of Sdh2, it interacts with Sdh6 and Sdh7, which serve to protect exposed Fe-S clusters during the assembly process (4) and further associates with a mature Sdh1 subunit forming a heterotetrameric assembly intermediate (5). Finally, the Sdh1-Sdh2 hydrophillic head docks to the IMM via interactions with the Sdh3-Sdh4 membrane anchor domain, which may or may not preassemble at the IMM (6). In the end, the concerted efforts of core subunits, dedicated assembly factors, and other ancillary factors facilitate the stepwise assembly of SDH.