Fig. S5.
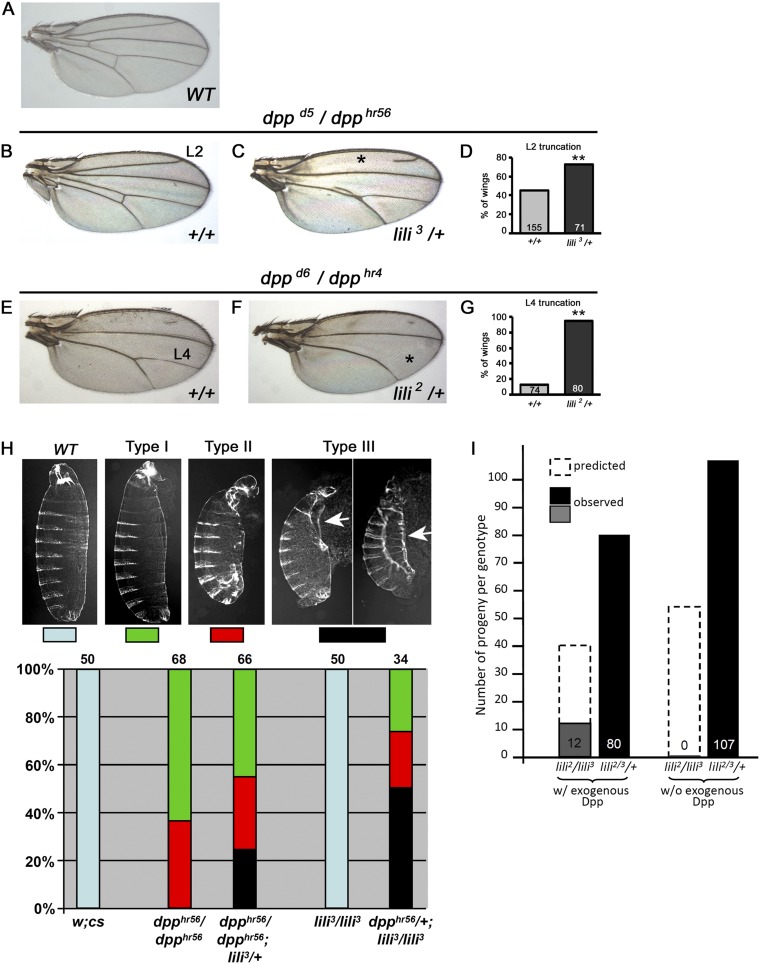
lili functions in other BMP-signaling contexts. (A–H) Hypomorphic loss-of-function dpp alleles provide sensitized genetic backgrounds useful in querying the contribution of other factors to various BMP-signaling events. lili mutant alleles interact with Dpp signaling in the wing (A–G) and in the embryo (H). (A–G) Lili contributes to Dpp signaling during wing vein development. The reduction of Dpp signaling caused by the dppd5/dpphr56 and dppd6/dpphr4 genotypes results in wing vein patterning defects without affecting proliferation of the wing pouch. These phenotypes are sensitive to a reduction in the level of other BMP signaling components, such that heterozygosity increases the severity of vein defects (29, 30). (A) A WT adult wing. (B and C) In wings from dppd5/dpphr56 adults, the L2 vein is sometimes truncated (*) and the intervein region between L4 and L5 is sometimes reduced or lost. (C and D) Introducing one copy of lili3 in this background enhances the severity of both phenotypes. Quantification of L2 truncation is shown in D. (E) In the more severe dppd6/dpphr4 allelic combination, the L2 vein and L4–L5 intervein are mostly lost, but the L4 vein remains largely intact. (F) Introducing one copy of the lili2 mutant allele dramatically enhances the severity of the L4 loss (*); quantified in G. (H) A genetic interaction between dpp and lili mutant alleles is also observed in the embryo resulting in the enhancement of cuticle defects. All cuticles are shown with anterior on the top and ventral to the left. The embryonic lethal dpphr56/dpp hr56 genetic background produces a range of phenotypes, from cuticles with eight abdominal segments and variable deformities of the head and filtzkorper (Type I) to cuticles with seven or fewer abdominal segments, severe head defects, and internalization or complete loss of filtzkorper (Type II) (31). When one copy of WT lili is also removed (dpphr56/dpphr56; lili3/+), the range of mutant phenotypes becomes more severe, including embryos displaying the type III pattern (type II-like but with failure of dorsal closure; a process known to be dpp-dependent; ref. 32). Arrows point to failed dorsal closure; least and most severely affected cuticle are shown. Conversely, introduction of one dpphr56 allele in a lili3 homozygous background has severe consequences. Whereas all homozygous lili3/lili3 embryos and the large majority of heterozygous dpphr56/+ hatch with an essentially normal cuticle pattern (a small fraction, few %, of dpphr56/+ embryos die with anterior defects; ref. 31), lili3/lili3; dpphr56/+ embryos die with moderate to severe abnormalities including failure of dorsal closure. In these experiments, double heterozygous dpphr56/+; lili3/+ cuticles were indistinguishable from dpphr56/+; this result is not surprising because lili has a strong maternal component that likely masks its embryonic role in Dpp signaling unless the pathway is more severely compromised. These findings show that Lili contributes to BMP signaling during embryonic development as well. (I) All homozygous and transallelic lili-mutant combinations are 100% larval lethal. Leaky expression of Dpp in a hs-Gal4 UAS-dpp background (without heat shock) results in substantial rescue of the larval lethality. Numbers shown are total heterozygous adults (lili2 or 3/+ = lili2 or 3/TM6B) compared with total lili2/lili3 adults. In these crosses, the approximate expected ratio is 2:1 for lili2 or 3/TM6B to lili2/lili3, in the case of full rescue. Although early larval lethality has not been reported for any class of dpp alleles (33), the considerable rescue achieved by exogenous Dpp (∼1/3 of expected progeny) suggests that lili2/lili3 lethality is, at least in part, due to a dpp-dependent process.