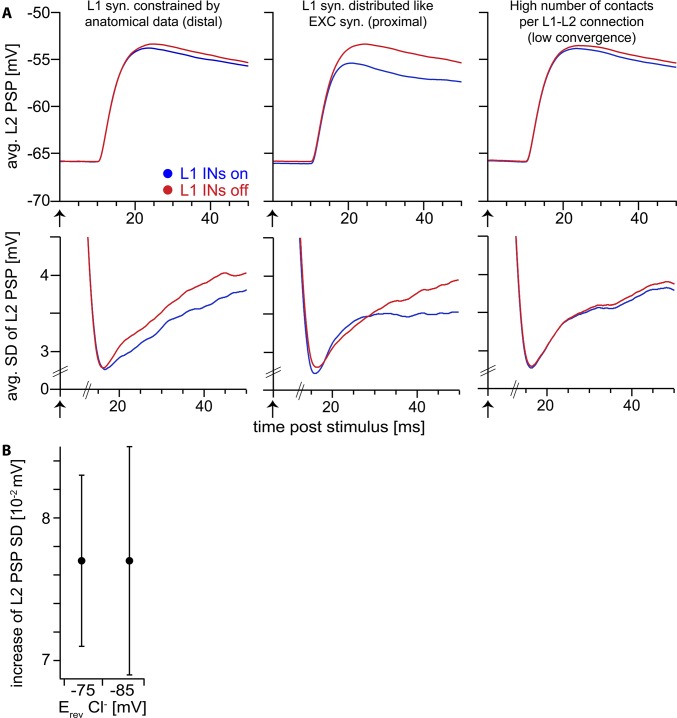
Fig. S3.
Sensitivity analysis of the computational model. (A) Top row: whisker-evoked PSP of L2 PN model averaged across 100,000 simulation trials. Bottom row: SD of whisker-evoked PSP across 100,000 simulation trials. (Left) Spatial distribution of L1 IN synapses on L2 PN constrained by anatomical data (Fig. 3). (Center) L1 IN synapses on L2 PN distributed spatially overlapping with excitatory synapses of PNs (i.e., located on proximal dendrites; mean distance to soma along dendrites 97 ± 45 µm). In this scenario, L1 INs affect both PSP amplitude and SD at the soma. These observations are in line with the detailed explanation of the mechanism (Fig. 4 D and E and Fig. S4): Proximal inhibitory synapses affect NMDAR-mediated nonlinearities. At the same time, the shunt level (SL) in this configuration extends to the soma and affects the membrane potential at the soma, in contrast to the anatomically constrained configuration in the left panel (i.e., IPSPs of proximal L1 INs are visible at the soma). This scenario was not observed in the in vivo experiments, suggesting that distal inhibition of L2 PNs by L1 INs is a general organizational principle of this circuit. (Right) To test our assumption of high convergence of L1 IN inputs onto the L2 PN (i.e., L1 IN synapses originate from a large fraction of the presynaptic population), we generated network configurations with high specificity (i.e., low convergence) of distal L1 IN to L2 PN contacts (here: 11 synapses per connection). A high number of synapses per connection are equivalent to a low number of connected presynaptic L1 INs. In combination with the low spiking probability of L1 INs, this results in a small number of trials in which inhibitory synapses are activated. Therefore, the effect of L1 INs (reduction of PSP SD) is largely abolished. Because we observed reduction of PSP SD in every L2 PN recorded in vivo (Fig. 5E), this suggests the assumption of high convergence/low specificity of the L1 IN–L2 PN connection is justified. Furthermore, this result is in line with previous observations, indicating that multiple IN synapses need to be active to maximize the global effect of distal dendritic shunting (20). (B) A hyperpolarized chloride reversal potential (−85 mV) has no effect on the reduction of the PSP SD by L1 IN synapses. The absolute value of the PSP SD is largely unaffected (control: 3.39 ± 0.04 mV, hyperpolarized: 3.34 ± 0.04 mV).